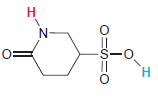
In each compound below, two protons are clearly identified. Determine which of the two protons is more
Question:
(a)
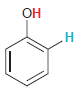
(b)
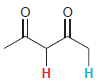
(c)
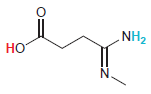
(d)
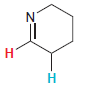
(e)
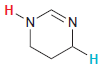
(f)
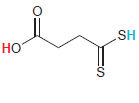
(g)
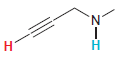
(h)
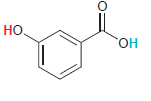
(i)
Transcribed Image Text:
Он Н н н
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 90% (10 reviews)
a b ...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which of the following compounds is more easily decarboxylated? CH2CH2 or
-
Which of the following compounds is more likely to exhibit activity as a tranquilizer? CH3 CH CH O or CH C
-
Identify which of the following compounds is more acidic and explain your choice.
-
(a) Show that (x) = 2x + 3x 36x is not one-to-one on (-, ). (b) Determine the greatest value c such that is one-to-one on (-c, c).
-
Look up the company's mission and guiding principles at the company's Web site. What do you think of the mission and guiding principles? Describe how these would influence how a batiste at a local...
-
The following item may contain errors in grammar, capitalization, punctuation, abbreviation, number style, word division, and vocabulary. Rewrite each sentence, correcting all errors. If a sentence...
-
Design an experiment. Use a diagram to outline the design of the experiment in this medical study. Part I Review Exercises 187
-
Natalie has been approached by Ken Thornton, a shareholder of The Beanery Coffee Inc. Ken wants to retire and would like to sell his 1,000 shares in The Beanery Coffee, which represents 20% of all...
-
Imperial Jewelers manufactures and sells a gold bracelet for $ 4 0 6 . 0 0 . The company's accounting system says that the unit product cost for this bracelet is $ 2 6 3 . 0 0 as shown below: \ table...
-
Frequency analysis of amplitude-modulated discrete-time signal the discrete-time signal x(n) = cos2 f 1 n + cos2 f 2 n Where f 1 = 1/18 and f 2 = 5/128, modulates the amplitude of the carrier x 0 (n)...
-
Amines contain C-N single bonds, while imines contain C - N double bonds: :N Amine Imine
-
For each pair of compounds below, predict which will be more acidic: (a) HCl HBr (b) H 2 O H 2 S (c) NH 3 CH 4 (d) (e) Cl,C Cl3
-
Peter is a fudge maker of some renown and obviously requires a reliable supplier of sugar. His current sugar supplier has been very dependable but recently, is delivering latesometimes days at a...
-
Please help! I'm stuck 1) What purpose would your computer system serve? Business or personal or both? 2) Is this laptop/portable or desktop with monitor attached or all-in-one desktop? 3) What would...
-
The airline industry is severely hit by the COVID-19. Rows 6 to 85 show the daily closing prices of three stocks (i.e.,Qantas Airways Limited (QAN.AX), Singapore Airlines Limited (C6L.SI), and Cathay...
-
Using C+ Write a program to let users input two integers. If the first number is greater than the second number, print "The first number is larger". If the second number is greater than the first...
-
7. The normal model Show that if the risk-neutral distribution of ST is given by ST | S ~N (F, (T-t)), where F = F(t, T)istheforwardprice, thenthepriceofa K-strike straddle is approxim- ated by Z(t,...
-
25 cm 75 cm Water Parabola 2. The wheel-well of a custom truck-mounted water tank has a semi- parabolic shape as shown (assume point A corresponds to the peak). It's width is projected 150 cm into...
-
Solve each system. -5x + 2y + z = 5 -3x - 2y 2 = 3 -x+6y= 1
-
A spacecraft has left the earth and is moving toward Mars. An observer on the earth finds that, relative to measurements made when the spacecraft was at rest, its a. length is shorter b. KE is less...
-
Predict the major products of the following reactions: (a) (b) (c) cat. H2SO H,O (2) H202. NaOH (1) Hg(OAc), H2O/THF (2) NaBH4, NaOH
-
Show how you might prepare 2-bromobutane from (a) 2-Butanol (b) 1-Butanol (c) 1-Butene (d) 1-Butyne
-
Starting with 2-methylpropene (isobutylene) and using any other needed reagents, outline a synthesis of each of the following (T = tritium, D = deuterium): (a) (b) (c) (d)
-
(15 points) Stressed $2.500,000 of S% 20 year bands. These bonds were issued Jary 1, 2017 and pay interest annually on each January 1. The bonds yield 3% and was issued at $325 8S! Required (2)...
-
Packaging Solutions Corporation manufactures and sells a wide variety of packaging products. Performance reports are prepared monthly for each department. The planning budget and flexible budget for...
-
1. A company issued 10%, 10-year bonds with a par value of $1,000,000 on January 1, at a selling price of $885,295 when the annual market interest rate was 12%. The company uses the effective...

Study smarter with the SolutionInn App


