In each of the following cases, identify whether the reagent shown is suitable to accomplish the task
Question:
(a) To protonate
using H2O
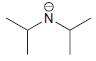
(b) To protonate
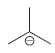
using
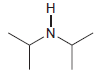
(c) To deprotonate
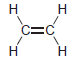
using
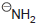
(d) To protonate
using H2O
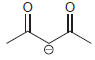
(e) To protonate
using H2O
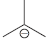
(f) To protonate
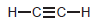
using
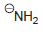
Transcribed Image Text:
OZ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
a Yes because a negative charge on an oxygen atom will be more stable than a negative ch...View the full answer

Answered By

Muhammad adeel
I am a professional Process/Mechanical engineer having a vast 7 years experience in process industry as well as in academic studies as a instructor. Also equipped with Nebosh IGC and lead auditor (certified).
Having worked at top notch engineering firms, i possess abilities such as designing process equipment, maintaining data sheets, working on projects, technical biddings, designing PFD and PID's etc.
Having worked as an instructor in different engineering institutes and have been involved in different engineering resrearch projects such as refinery equipment designing, thermodynamics, fluid dynamics, chemistry, rotary equipment etc
I can assure a good job within your budget and time deadline
4.90+
52+ Reviews
60+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In each of the following cases, calculate the nominal exchange rate assuming purchasing power parity holds. a. A bottle of wine sells for $16 in the United States and 10 in France. b. A book sells...
-
Write balanced net ionic equations for the reactions that occur in each of the following cases. Identify the spectator ion or ions in each reaction. (a) Cr2 (SO4)3(aq) + (NH4)2CO3(aq) (b) Ba...
-
In each of the following cases draw the structure of an alkyl halide that will undergo an E2 elimination to yield only the indicated alkene. a. b. c. d. ? = E2 E2
-
In Exercises show that the function y = (x) is a solution of the differential equation. y = 4e-x y" - y = 0
-
In the Continuing Payroll Problem A, presented at the end of succeeding chapters, you will gain experience in computing wages and salaries and preparing a payroll register for Kipley Company, Inc., a...
-
Use the laws of exponents to simplify the algebraic expressions. Your answer should not involve parentheses or negative exponents. 2x /x
-
Percents up and down. Between March 2007 and June 2007 the average price of regular gasoline increased from $2.40 per gallon to $3.00 per gallon. (a) Verify that this is a 25% increase in price. (b)...
-
Yolanda is a cash basis taxpayer with the following transactions during the year: Cash received from sales of products........... $65,000 Cash paid for expenses (except rent and interest)..........
-
On January the end of the Test weekly pay pead of the year. Reges Company employees and 522.760 offices and $65,340 of sales sales wings from the mioyees les include RCA Social Security of the role...
-
You are a management consultant for a 30-year old partner in a large law firm. In a meeting, your client says: According to an article in the New York Times, 57 percent of large law firms have a...
-
As we will learn in Chapter 21, treating a lactone (a cyclic ester) with sodium hydroxide will initially produce an anion: This anion rapidly undergoes an intramolecular proton transfer, in which the...
-
Identify and name the parent in each of the following compounds: (a) (b) (c) (d) (e) (f) (g) (h) (i)
-
What is a conversion? What are some examples of conversions on an e-commerce website? Appendix
-
Global Operations Management is supported by Strategic Supply Chain Management in many ways. Elucidate the following; List and briefly define/describe the Five (5) Components of Strategic Supply...
-
The Alpine House, Inc. is a large winter sports equipment broker. Below is an income statement for the company's ski department for a recent quarter. LA CASA ALPINA, INC. Income Statement - Ski...
-
Two investment portfolios are shown. Investment Portfolio 1 Portfolio 2 ROR Savings Account $1,425 $4,500 2.80% Government Bond $1,380 $3,600 1.55% Preferred Stock $3,400 $2,150 11.70% Common Stock...
-
The following information pertains to JAE Corporation at January 1, Year 1: Common stock, $8 par, 11,000 shares authorized, 2,200 shares issued and outstanding Paid-in capital in excess of par,...
-
Group dynamics are important elements within the leading facet of the P-O-L-C framework. Discuss a time in your professional, school, or personal life when you experienced the Five Stages of Group...
-
Point A has coordinates (-2, 6) and point B has coordinates (4, -2). What is the standard form of the equation of line AB?
-
What can scientists learn by comparing the fossilized skeletons of extinct primates with the bones of modern species?
-
Propose a reasonable mechanism for the following reaction. OH
-
Propose a reasonable mechanism for the following reaction. OH cat. H PO EtOH
-
Vicinal halo alcohols (halohydrins) can be synthesized by treating epoxides with HX. (a) Show how you would use this method to synthesize 2-chlorocyclopentanol from cyclopentene. (b) Would you expect...
-
1,600 Balance Sheet The following is a list (in random order) of KIP International Products Company's December 31, 2019, balance sheet accounts: Additional Paid-In Capital on Preferred Stock $2,000...
-
Question 3 4 pts 9 x + 3 x 9 if x 0 Find a) lim f(x), b) lim, f(x), C), lim , f(x) if they exist. 3 Edit View Insert Format Tools Table : 12pt M Paragraph B IV A2 Tv
-
Mr. Geoffrey Guo had a variety of transactions during the 2019 year. Determine the total taxable capital gains included in Mr. Guo's division B income. The transactions included: 1. On January 1,...

Study smarter with the SolutionInn App


