Lactones can be prepared from diethyl malonate and epoxides. Diethyl malonate is treated with a base, followed
Question:
![1) NaOEt EtO EtO OEt 2) OEt Н,о Heat [H,O*] Н,о* Heat Но Но ОН (-CO2) ОН ОН](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1525/0/6/8/3845ae6b260351221525068355373.jpg)
Using this process, identify what reagents you would need to prepare the following compound:
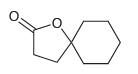
Transcribed Image Text:
1) NaOEt EtO EtO OEt 2) OEt Н,о Heat [H,O*] Н,о* Heat Но Но ОН (-CO2) ОН ОН
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (10 reviews)
Eto OE...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Outline syntheses of each of the following from diethyl malonate and any other required reagents: (a) 2-Methylbutanoic acid (b) 4-Methyl-1-pentanol (c) (d) OH
-
Thiols can be prepared from the reaction of thiourea with an alkyl halide, followed by hydroxide-ion-promoted hydrolysis. a. Propose a mechanism for the reaction. b. What thiol would be formed if the...
-
When ethylene oxide is treated with a strong nucleophile, the epoxide ring is opened to form an alkoxide ion that can function as a nucleophile to attack another molecule of ethylene oxide. This...
-
aj b) If the magnitude of the force F is 700 N, what is the value of the internal moment at point A in N.m? d) 0.5 m 540 180 210 A 0.8 m 240 Bo birak F A
-
For the composite wall idealized by the one-dimensional model shown in Figure P13-7, determine the interface temperatures. For element 1, let Kxx = 5 W (m ( oC) for element 2, Kxx = 10 W (m ( oC);...
-
You are a consultant hired to provide guidance for a family-owned small sized accounting and financial consulting service business with their employees based in Vancouver, B.C. The organization earns...
-
Add the beta coefficients to the initial table. Stock P/E P/B P/S PEG beta Is there a relationship between the betas and each ratio? A low beta and a low valuation ratio may suggest that the stock is...
-
During March 19X1 the West Tacoma Works of Ryan Industries was considering the purchase of a 100-ton crane to be used to move sub assemblies around the shop floor and to transport finished products...
-
B. Information for two retailers for 2019 appears below: Retailer A 39.0 Retailer B 50.5 Days' purchases in accounts payable Retailer A's net sales and merchandise purchases during 2019 were $91,250...
-
The following is a series of annual sales (in $ millions) over an 11- year period ( 2003 to 2013): a. Construct a time- series plot. b. Does there appear to be any change in annual sales over time?...
-
Draw the structure of the product with molecular formula C 10 H 10 O that is obtained when the compound below is heated with aqueous acid. CN CN C10H100 Heat
-
Predict the major product of the following transformation. CO2ET C10H100 Heat
-
For each of the following unrelated situations, calculate the annual amortization expense and prepare a journal entry to record the expense: a. A patent with a 10 -year remaining legal life was...
-
(b) A cylindrical storage tank with base area 90 m is being filled with water through an entry duct with cross-section area 250 cm, as shown in Figure 3. Concurrently, water is being extracted from...
-
Po A cylinder/piston arrangement contains 5 kg of water at 100 C with x= 20%. Initially the piston of mass m, 75 kg rests on a set of stops (see figure). The outside pressure is 100 kPa, and the area...
-
TABLE 2 Present Value of an Annuity of $1 n 123456 8% 9% 0.925926 0.917431 4 7 8 9 10 11 12 13 14 15 16 17 11.652296 10.837770 10.105895 9.446649 12.165669 11.274066 10.477260 9.763223 18 19...
-
There are 4 suits (heart, diamond, clover, and spade) in a 52-card deck, and each suit has 13 cards. Suppose your experiment is to draw one card from a deck and observe what suit it is. Express the...
-
Write isotopic symbol of zirconium and how many neutrons are present in one atom of this isotope
-
Let A = {1, 2, 3, 4, 5, 6}, B = {1, 3, 5}, C = {1, 6}, and D = {4}. Find each set. B C
-
For each equation, (a) Write it in slope-intercept form (b) Give the slope of the line (c) Give the y-intercept (d) Graph the line. 7x - 3y = 3
-
Outline a synthesis for each of the following compounds from the indicated starting material and any other reagents. 4-methyl-3-nitropyridine from - lpicoline
-
Predict the predominant product in each of the following reactions. Explain your answer. 3,4-dibromopyridine + NH3, heat (C5H5BrN2)
-
Using bases (B:) and acids (+BH) as needed, provide a curved-arrow mechanism for the conversion of the c-amino acid serine into formaldehyde and glycine (Eq. 25.53, p. 1242). Eq. 25.53 formaldehyde...
-
Show that the convexity for a zero coupon bond with m payments per year is (m) n(n + -)(1+ m m
-
Abdul Canarte , a Central Bank economist, noticed that the total group purchasing basket of goods (CPI) has gone from $149,740.00 to $344,460.00 in 8 years. With monthly compounding, what is the...
-
ABC Corporation expects sales next year to be $50,000,000. Inventory and accounts receivable (combined) will increase $8,000,000 to accommodate this sales level. The company has a profit margin of 6...

Study smarter with the SolutionInn App


