Nitriles undergo alkylation at the α position much like ketones undergo alkylation at the α position. The
Question:
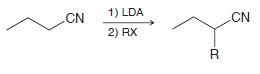
The α position of the nitrile is first deprotonated to give a resonance- stabilized anion (like an enolate), which then functions as a nucleophile to attack the alkyl halide.
(a) Draw the mechanism for this process.
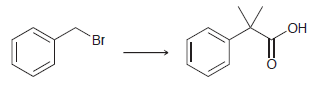
(b) Using this process, show the reagents you would use to achieve the following transformation:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





