Question: Predict the expected product(s) when benzene is treated with each of the following alkyl halides in the presence of AlCl 3 . In each case,
Predict the expected product(s) when benzene is treated with each of the following alkyl halides in the presence of AlCl3. In each case, assume conditions have been controlled to favor monoalkylation
a.
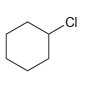
b.
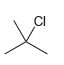
c.
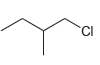 .
.
.CI CI
Step by Step Solution
3.33 Rating (180 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


