Predict the major product of each reaction below: (a) (b) (c) 1) EtMgBr 2) H20 :? H.
Question:
(a)
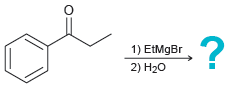
(b)
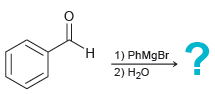
(c)
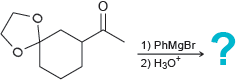
Transcribed Image Text:
1) EtMgBr 2) H20 :? H. 1) PhMgBr 2) H20
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
a...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict the major product of each reaction below: (a) (b) (c) RCO3H RCO, ? H RCO3H
-
Predict the major product of the following reaction, and give a mechanism to support your prediction. NBS, h CH,CH ethylbenzene
-
Predict the major product for each reaction below: (a) (b) 1) [H'), HS 2) Raney Ni SH 1) [H*), HS' `H. 2) Raney Ni SH
-
Prove that there exist two languages A and B that are Turing-incomparablethat is, where A T B and B T A.
-
One of your corporate clients has approached you about whether or not its employees are required to include certain benefits provided by the corporation in their income. In particular, the...
-
What automated control can help prevent the following risk, "Customer data is incorrect in the customer database
-
How are choiceboard and personalization systems used in the PizzaHut.com website?
-
From a random sample of 36 business days from February 24, 2016, through February 24, 2017, the mean closing price of Apple stock was $116.16. Assume the population standard deviation is $10.27. You...
-
Cost of Equity 4. Problem 10.04 (Cost of Equity with and without Flotation) eBook 1 Problem Walk-Through Jarett & Sons's common stock currently trades at $32.00 a share. It is expected to pay an...
-
A Wall Street Journal article recently asked readers the following questions. What's your answer? a. An accident has caused deadly fumes to enter the school ventilation system where it will kill five...
-
When 2 moles of benzaldehyde are treated with sodium hydroxide, a reaction occurs in which 1 mole of benzaldehyde is oxidized (giving benzoic acid) while the other mole of benzaldehyde is reduced...
-
Identify the reagents necessary to accomplish each of the transformations below: (a) (b) Me
-
Describe the characteristics of arrivals, waiting lines, and service systems 784
-
Question 37 Plantito Inc., produces potted plants. For next year, Pietro predicts that 45,000 units will be produced, with the following total costs: Direct materials Direct labor ? 80,000 Variable...
-
When you are to design a data transmission system, you have two key considerations to work with: data rate and distance, with emphasis placed on achieving the highest data rates over the longest...
-
How much work does a supermarket checkout attendant do on a can of soup he pushes 0.600 m horizontally with a force of 5.00 N? Express your answer in joules and kilocalories. 3 . (a) Calculate the...
-
Suppose in its income statement for the year ended June 30, 2022, The Clorox Company reported the following condensed data (dollars in millions). Salaries and wages expenses$460 Research and...
-
Consider the extensive form game show in the figure below. How many strategies does Player 2 have in this game? (2,2,1) b (2,4,2) 03 by 03 02 dz (4.2,0) (2.0.2) (0.3.4) (3,5,3) (3,1,2)
-
Determine whether the given ordered pair is a solution of the given system. = 8 2xy 3x + 2y = 20 ; (5,2)
-
I frequently use NY Times and CNN and am aware of Fox News but I never use it. I visit these sites, NY Times and CNN, a few times a week whenever I have to research something or see something on...
-
Malonic acid, HO2CCH2CO2H, is a diprotic acid. The pKa for the loss of the first proton is 2.83; the pKa for the loss of the second proton is 5.69. (a) Explain why malonic acid is a stronger acid...
-
At 25oC the enthalpy change, Ho, for the ionization of trichloroacetic acid is +6.3 kJ mol-1 and the entropy change, So, is +0.0084 kJ mol-1 K-1. What is the pKa of trichloroacetic acid?
-
Use the curved-arrow notation to write the reaction that would take place between dimethylamine (CH3)2NH and boron trifluoride. Identify the Lewis acid, Lewis base, nucleophile, and electrophile and...
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...
-
John has to choose between Project A and Project B, which are mutually exclusive. Project A has an initial cost of $30,000 and an internal rate of return of 16 percent. Project B has an initial cost...
-
Complete the table below, for the above transactions

Study smarter with the SolutionInn App


