Predict which compound will react more readily in an S N 1 process, and explain your choice.
Question:
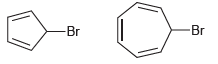
Transcribed Image Text:
Br -Br
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
The first step of an S N 1 process is loss of a leaving group forming a carbocation so we comp...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
CuI, CsI, and NaI each adopt a different type of structure. The three different structures are those shown in Figure 12.26. (a) Use ionic radii, Cs+ (r = 1.81 ), Na+ (r = 1.16 ), Cu+ (r = 0.74 ), and...
-
For each pair of compounds below, predict which compound will have the higher boiling point and explain your choice: a) CH 3 CH 2 CH 2 OCH 3 or CH 3 CH 2 CH 2 CH 2 OH b) CH 3 CH 2 CH 2 CH 3 or CH 3...
-
For each of the following groups of compounds, identify which compound will react most rapidly with ethyl chloride in the presence of aluminum trichloride. Explain your choice in each case, and then...
-
Determine the products of inertia of the uniform slender rod of mass m about the coordinate axes shown. h b
-
Please answer the following questions related to the article provided and submit your paper to the assignment link. Please ensure that you list the article on your References page, and use in-text...
-
Rise of the hackers essay prompt question Do you think you might be vulnerable to the social engineering attack that hackers used against Matt in the video?
-
Expatriate managers need to be equipped with up-to-date business and technological knowledge. But to be successful in their assignments, they also need to acquire a sound knowledge of local...
-
Your firm has debt worth $200,000, with a yield of 10 percent, and equity worth $400,000. It is growing at a seven percent rate, and faces a 40 percent tax rate. A similar firm with no debt has a...
-
Clark's Chensical Company recelved refundoble deposits on returnable containers in the omount of $105.000 during 2024 . Thirteen percent of the containers were not returned. The deposits are boted on...
-
Karen Kluster opened Lube and Wash on January 1, 2016. The business is subject to FICA taxes. At the end of the first quarter of 2016, Kluster, as president of the company, must file Form 941,...
-
Explain the vast difference in pK a values for the following two apparently similar compounds. pk, = 16 pk, = 36
-
Identify which of the following compounds is more acidic and explain your choice.
-
Refer to the information in Exercise 6.30. Information in Exercise 6.30. Reliable Fittings had 6,000 units 60% complete for conversion costs in beginning WIP. During the month 20,000 units were...
-
This case study is based on a fictional character on NBC's The Office. Michael is the central character of the series, serving as the Regional Manager of the Scranton branch of a paper distribution...
-
What is the significance of a balance sheet in understanding a firm's financial position? How do changes on the right side of the balance sheet (liabilities and equity) impact a company's financial...
-
A current event analysis where the article must focus on a management concepts). You will read the article and then provide an analysis of the subject matter discussed. The article should complement...
-
Given an exponential distribution with =20, what is the probability that the arrival time is a. less than X=0.2? b. greater than X = 0.2? c. between X=0.2 and X 0.3? d. less than X=0.2 or greater...
-
Choose at least two measures of employee attitudes. Discuss them and tell me about your discussion. Which group you believe are the most effective and efficient measures? Why? 2) Discuss turnover,...
-
Solve each system by graphing. = 2x + y = 4 3x-y = 6
-
In what ways does a well-designed enterprise search software vary from popular search engines (e.g., Bing, DuckDuckGo, and Google)?
-
Unknown Y has a molecular formula of C3H6O2. It contains one functional group that absorbs infrared radiation in the 3200-3550-cm-1 region (when studied as a pure liquid; i.e., "neat"), and it has no...
-
Which atoms in each of the following molecules are chirality centers? (a) (b) (c) (d) OH OH Lactic acid Glyceraldehyde OH HO 0 0 OH Ascorbic acid (vitamin C) OH HO Estradiol (an estrogen)
-
Write three-dimensional formulas and designate a plane of symmetry for all of the achiral molecules in Practice Problem 5.4. (In order to be able to designate a plane of symmetry you may need to...
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


