Propose a plausible mechanism for the following transformation. Ph H,O* Ph
Question:
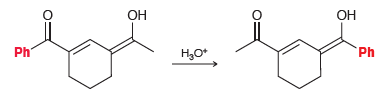
Transcribed Image Text:
ОН ОН Ph H,O* Ph
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (18 reviews)
O D Ph 64 ...View the full answer

Answered By

Rinki Devi
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions.
Hi there! Are you looking for a committed, reliable, and enthusiastic tutor? Well, teaching and learning are more of a second nature to me, having been raised by parents who are both teachers. I have done plenty of studying and lots of learning on many exciting and challenging topics. All these experiences have influenced my decision to take on the teaching role in various capacities. As a tutor, I am looking forward to getting to understand your needs and helping you achieve your academic goals. I'm highly flexible and contactable. I am available to work on short notice since I only prefer to work with very small and select groups of students.
I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and helped them achieve great subject knowledge.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw a plausible mechanism for the following transformation: , [H2SO4]
-
Draw a plausible mechanism for the following transformation: -NH2 [H,SO] NH2 [-H,0]
-
Draw a plausible mechanism for the following transformation ,*
-
Calculate the anion gap for a 58 year old insulin dependent diabetic woman who is admitted to the emergency room in a comatose state Na-135mmol/L K= 3.5 mmol/L CI-102 mmol/L HCO3-= 15mmol/L. Include...
-
The radio control car front steering unit in Figure P13-46 (detailed dimensions shown in Figure P11-22) is now relieved of stress, but its base has an applied temperature of 100 oF. The lower surface...
-
Requirements: APA with in-text and reference citations. explain the issue of common and civil law system internationally. intellectual property, copyright, and legal ramifications violation of human...
-
You purchased $1,000 of IBM stock at the end of each quarter from 2000 through 2006. Excluding commissions, how many shares have you accumulated? As of January 2010, IBM was selling for $130. What...
-
Frobisher Inc. (Frobisher) uses the lower of cost and NRV rule to value its inventory. Frobishers inventory on February 28, 2017 had a cost of $1,125,000 and a NRV of $1,035,000. Required: a. By how...
-
Symon's Suppers Co. has announced that it will pay a dividend of $4.45 per share one year from today, Additionally, the company expects to increase Its dividend by 3.9 percent annually. The required...
-
(a) How do the components of a vector transform under a translation of coordinates (x = x, y = y ?? a, z = z, Fig. 1.16a)? (b) How do the components of a vector transform under an inversion of...
-
Consider the structures of the constitutional isomers, Compound A and Compound B (below). When treated with aqueous acid, Compound A undergoes isomerization to give a cis stereoisomer. In contrast,...
-
Propose a plausible mechanism for the following transformation. NaOH, H20 Heat
-
Make the given changes in the indicated examples of this section, and then solve the resulting problems. In Example 5(b), change the exponent 3 to 2. Data from Example 5(b) (2a - b) = (2a - b)(2a -...
-
In the exchange lemma for the scheduling problem, we say that the first event to finish a* in a given time period [i,j] is always part of the optimal solution for that same time period. To argue...
-
4 10 points Company's year-end is December 31. Calculate depreciation for each year of the machine's estimated useful life under each of the following methods: (Do not round intermediate...
-
3. The walls of an oven are made from steel sheets with insulating board between them of thermal conductivity 0.18 J m-1 s -1 C-1 . If the maximum internal temperature in the oven is 300C and the...
-
Egyptian Spa produces two different spa products: Relax and Refresh. The company uses three operations to manufacture the products: mixing, blending, and packaging. Because of the materials used,...
-
Part A At a given instant A has the motion shown in (Figure 1). Determine the acceleration of B at this instant. Express your answer in feet per second squared to three significant figures. Enter...
-
Two sets are specified by graphs. Graph the intersection of the two sets. 0 0 5 5
-
Let (x) = x 2 - 9, g(x) = 2x, and h(x) = x - 3. Find each of the following. (((--) 2
-
Give the principal organic product(s) expected when 2- methylpyridine or other compound indicated reacts with each of the following reagents. (a) Diliute aqueous NaOH (b) HNO3, H2SO4, heat; then -OH...
-
Rank the following compounds in order of increasing reactivity toward nitration with HNO3 and explain your choices: thiophene, benzene, 3-methylthiophene, and Z-methvlfuran.
-
Rank the following compounds in order of increasing reactivity toward nitration with HNO3 and explain your choices: thiophene, benzene, 3-methylthiophene, and Z-methvlfuran.
-
Phantom Consulting Inc. is a small computer consulting business. The company is organized as a corporation and provides consulting services, computer system installations, and custom program...
-
Sam owns a 25% in Spade, LLC. In 2021, Spade reports $100,000 or ordinary income. What is Sams qualified business income (QBI) deduction? answer is 5,000 but please show how to get it
-
crane Inc. common chairs currently sell for $30 each. The firms management believes that it's share should really sell for $54 each. If the firm just paid an annual dividend of two dollars per share...

Study smarter with the SolutionInn App


