Propose a plausible synthesis for each of the following transformations. a. b. c. d. e. CN
Question:
a.
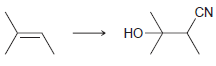
b.
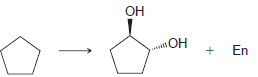
c.
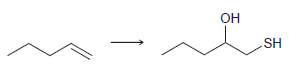
d.
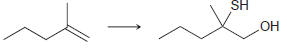
e.

Transcribed Image Text:
CN Но ОН Он En
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (13 reviews)
a b c d ...View the full answer

Answered By

Talha Talib
I am a member of IEEE society. As i am a student of electrical engineering badge 17 but beside of this i am also a tutor in unique academy. I teach calculus, communication skills, mechanics and economics. I am also a home tutor. My student Muhammad Salman Alvi is a brilliant A-level student and he performs very well in academics when i start to teach him. His weak point was mathematics but now he is performing well in mathematics. I am a scholarship holder in Fsc as i scored 1017 marks in metric out of 1100. Later on i got scholarship in Punjab Group of Colleges. I got 2nd position in robotics competition in 2018 as my project home automation select for the exhibition in Expocentre.
4.60+
23+ Reviews
62+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Propose a plausible synthesis for each of the following transformations: (a) (b) (c) (d) (e) (f) Br Br
-
Propose a plausible synthesis for each of the following transformations: (a) (b) (c) (d) H OCH3 NO2 Br OCH3 OCH3 Br NO2
-
Propose a plausible synthesis for each transformation. a. b. c. d. e. f. g. h. i. j. k. l. m. n. o. p. q. r. s. t. u.
-
Madison Company and Orwell Corporation are competitors. Compare the two companies by converting their condensed income statements to common-size statements. Which company earned more net income?...
-
Robert is a delivery specialist. He started at a young age by delivering newspapers. Later, he delivered pizzas. He has since purchased a van and he delivers electronics for a local electronic...
-
Perform the indicated operations. Leave the result in polar form. (50/236) (2/84) 125/47
-
Leadership: establishing direction, unity of purpose, and a supportive work environment? LO.1
-
Le Monde Company is a manufacturer of chemicals for various purposes. One of the processes used by Le Monde produces HTP3, a chemical used in hot tubs and swimming pools; PST4, a chemical used in...
-
One of the major controls over cash and cash transfers is to ensure that only authorised personnel are handling cash, making cash transfers, or investing excess cash. a. Electronic transfer of excess...
-
Given a database of the results of an election, find the number of seats won by each party. There are some rules to going about this: There are many constituencies in a state and many candidates who...
-
Predict the products for each of the following reactions. a. b. c. d. SH 1) NaOH Br 2) Br SNa
-
Ethylene glycol is one of the main components of automobile antifreeze. Using iodoethane as your starting material, show how you could prepare ethylene glycol.
-
If the elimination of volatile cash flows through risk management techniques does not significantly change a firm's expected future cash flows and WACC, investors will be indifferent to holding a...
-
4. (15pt) A group of students were asked if they have ever driven after drinking. They also were asked, "How many days per month do you drink at least two beers?" In the following discussion, 7 = the...
-
discuss how might you apply the concepts of Total Quality (TQ) to your personal and work environment. Consider your relations with others and your daily activities interactions with. Share the...
-
Dr. Bernstein wants to expand his radiology practice. Dr. Bernstein is researching various local banks for the best certificate of deposit rate to fund his expansion. One bank is willing to offer him...
-
An airplane is flying with a velocity of 240 m/s at an angle of 30.0 with the horizontal, as the drawing shows. When the altitude of the plane is 2.4 km, a flare is released from the plane. The flare...
-
Katsura Corporation incurred pre - operating costs: Investigatory expenses of $ 1 8 , 0 0 0 New employee training $ 2 5 , 0 0 0 Advertising $ 1 0 , 0 0 0 Land and building for use as a retail store...
-
Find the slope of each line, and sketch its graph. 2y = 3
-
MgO prevents premature evaporation of Al in a furnace by maintaining the aluminum as Al2O3. Another type of matrix modifier prevents loss of signal from the atom X that readily forms the molecular...
-
Write the equations for the remaining passages of the p-oxidation pathway following those shown infigure. CHCH2CH2CHH-CH2 CH2CH2CH2CH2 CH2CH2 CH-CSCOA Myristoyl CoA 8-Oxidation (passage 1)...
-
How many molecules of acetyl CoA are produced by catabolism of the following fatty acids, and how many passages of the a-oxidation pathway arc needed? (a) Palmitic acid, CH 3 (CH 2 ) 14 CO 2 H (b)...
-
Write a mechanism for the dehydration reaction of -hydroxybutyryl ACP to yield crotonyl ACP in step 7 of fatty-acid synthesis.
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


