Rank each of the bonds identified in order of increasing wave number. R-CEN - N-H - -
Question:
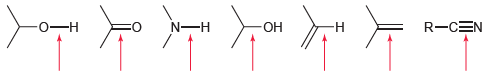
Transcribed Image Text:
R-CEN -о—н N-H -ОН -н
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (20 reviews)
OH NH In...View the full answer

Answered By

Grace Igiamoh-Livingwater
I am a qualified statistics lecturer and researcher with an excellent interpersonal writing and communication skills. I have seven years tutoring and lecturing experience in statistics. I am an expert in the use of computer software tools and statistical packages like Microsoft Office Word, Advanced Excel, SQL, Power Point, SPSS, STATA and Epi-Info.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Rank each of the following series of compounds in order of increasing oxidationlevel: CI (a) (b) CH3CN CH3CH2NH2 H2NCH2CH2NH2
-
Rank the following bonds in order of increasing ionic character: NOO, CaOO, COF, BrOBr, KOF.
-
The partial Lewis structure that follows is for a hydrocarbon molecule. In the full Lewis structure, each carbon atom satisfies the octet rule, and there are no unshared electron pairs in the...
-
Write the expression as one ratio without any negative exponents. x1/4x-3/4 X
-
Discuss the growing income inequality in the U.S. and the world with someone mature enough to have some perspective from experience about it. Ask them what they think about the widening gap between...
-
Simplify the given expressions. 6cos 3x sin 3x
-
13. What is the fixed rate in a 5-quarter interest rate swap with the first settlement in quarter 2?
-
Barra Concrete specializes in creating driveways and curbs for the residential market. Its accounting software uses exclusive OR (XOR) operations to convert the individual bits of a plaintext message...
-
Chenango Industries uses 1 0 units of part JR 6 3 each month in the production of radar equipment. The cost of manufacturing one unit of JR 6 3 is the following: \ table [ [ Direct material,$ 3 , 5 0...
-
Jimmy owns a garden in which he has planted N trees in a row. After a few years, the trees have grown up and now they have different heights. Jimmy pays much attention to the aesthetics of his...
-
All of the following compounds absorb IR radiation in the range between 1600 and 1850 cm -1 . In each case, identify the specific bond(s) responsible for the absorption(s), and predict the...
-
How would you distinguish between each pair of compounds in Problem 15.29 using IR spectroscopy? Problem 15.29 a. b. OH HO. HO m/z = 126.0315 m/z = 126.1404
-
How might a diet that is high in fiber also prevent a person from overeating?
-
C 2 H 6 O 2 + NaOH + 6 H 2 O C 2 H 3 NaO 3 + O 2 + 3 H 2Hydrogen is produced at the cathode, oxYGEN AT THE ANODE .Mass balance to produce 5000 tonnes a year of glycolic acid, formic acid and oxalic...
-
Please answer: a discussion of the ethical issues involved. The court might not itself consider the ethics of the actions of the parties. However, I ask that you consider the ethics of the following:...
-
In Exercises 21-24, use these results from the "1-Panel-THC" test for marijuana use, which is provided by the company Drug Test Success: Among 143 subjects with positive test results, there are 24...
-
I need help for an assignment of a review on research on Virtual Education on study motivation and academic performance in university students. I am attaching a research article from a magazine to...
-
Shouldice Hospital in Canada is widely known for one thing-hernia repair! In fact, that is the only operation it performs, and it performs a great many of them. Over the past two decades this small...
-
Give the domain of the power function. Approximate f(3) to the nearest hundredth. 13/2 = 27
-
White Bolder Investments (WBI) You are an intern working for WBI, a large investment advisory services in Sydney. Among other regular customers, WBI has been providing advisory services for Jumbo...
-
The industrial synthesis of methyl tert-butyl ether involves treatment of 2-methylpropene with methanol (CH3OH) in the presence of an acid catalyst, as shown in the following equation. CH3 H3C H3C...
-
The standard free energy of activation (G++) for hydration of 2-methylpropene to 2-methyl-2-propanol (Eq. 4.41, p. 169) is 91.3 kJ mol-t (Zt 8 kcal mol-l;. The standard free energy G for hydration of...
-
The standard free energy of activation (G++) for hydration of 2-methylpropene to 2-methyl-2-propanol (Eq. 4.41, p. 169) is 91.3 kJ mol-t (Zt 8 kcal mol-l;. The standard free energy G for hydration of...
-
3. The nominal interest rate compounded monthly when your $7,000 becomes $11,700 in eight years is ________
-
An investor can design a risky portfolio based on two stocks, A and B. Stock A has an expected return of 21% and a standard deviation of return of 39%. Stock B has an expected return of 14% and a...
-
Advanced Small Business Certifica Drag and Drop the highlighted items into the correct boxes depending on whether they increase or decrease Alex's stock basis. Note your answers- you'll need them for...

Study smarter with the SolutionInn App


