Refer to the periodic table (Figure 2.15 or inside front cover) and answer the following questions. a.
Question:
Refer to the periodic table (Figure 2.15 or inside front cover) and answer the following questions.
a. What Group VA element is a metal?
b. What is the Group IIA element in Period 3?
Figure 2.15
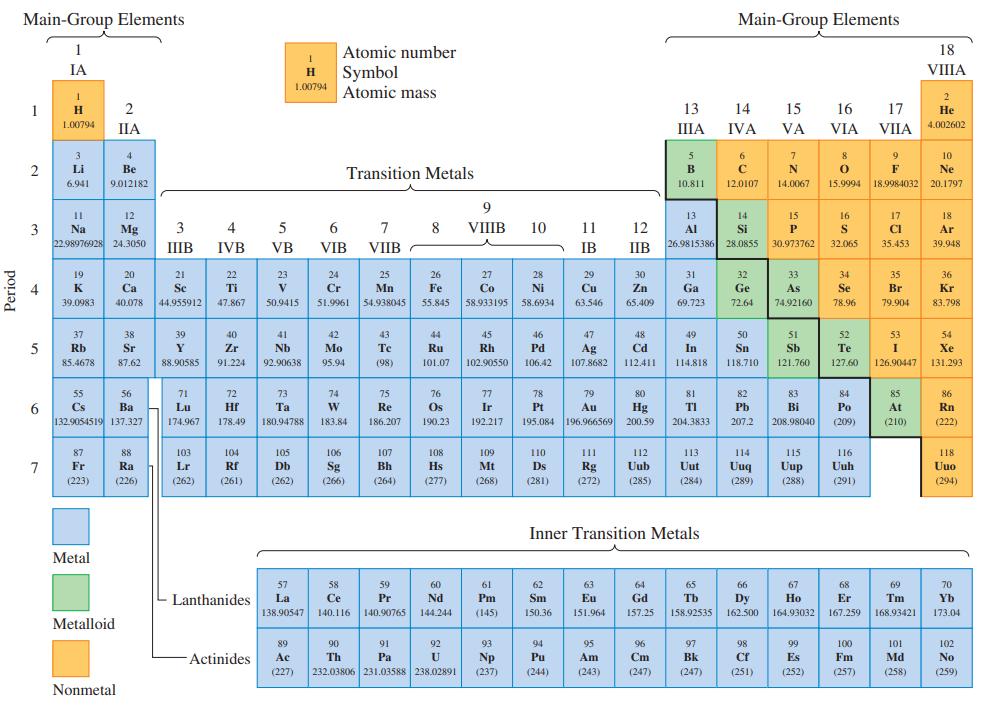
Transcribed Image Text:
Main-Group Elements Main-Group Elements 1 Atomic number 18 IA Symbol Atomic mass H VIIА 1.00794 1 13 14 15 16 17 Не 1.00794 ПА IIIA IVA VA VIA VIIA 4.002602 3 4 6 7 8 9 10 2 Li Be Transition Metals B F Ne 6.941 9.012182 10.811 12.0107 14.0067 15.9994 18.9984032 20.1797 12 13 14 15 16 17 18 3 22.98976928 24.3050 3 4 6 7 8 VIIIВ 10 11 12 26.9815386 28.0855 Na Mg Al Si P S CI Ar IIIB IVB VB VIB VIIB IB IIB 30.973762 32.065 35.453 39.948 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 4 K Са Se Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 39.0983 40.078 44.955912 47.867 50.9415 51.9961 54.938045 55.845 58.933195 58.6934 63.546 65.409 69.723 72.64 74.92160 78.96 79.904 83.798 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 Rb Sr Y Zr Nb Mo Те Ru Rh Pd Ag 107.8682 Cd In Sn Sb Те Xe 85.4678 87.62 88.90585 91.224 92.90638 95.94 (98) 101.07 102.90550 106.42 112.411 14.818 118.710 121.760 127.60 126.90447 131.293 55 56 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 Cs Ва Lu Hf Ta W Re Os Ir Pt Au Hg 200.59 TI Pb Bi Po At Rn 132.9054519 137.327 174.967 178.49 180.94788 183.84 186.207 190.23 192.217 195.084 196.966569 204.3833 207.2 208.98040 (209) (210) (222) 114 Uuq 87 88 103 104 105 106 107 108 109 110 II 112 113 115 116 118 Fr Ra Lr Rf Db Sg Bh Hs Mt Ds Rg Uub Uut Uup Uuh Uuo (223) (226) (262) (261) (262) (266) (264) (277) (268) (281) (272) (285) (284) (289) (288) (291) (294) Inner Transition Metals Metal 57 58 59 60 61 62 63 64 65 66 67 68 69 70 Lanthanides La Ce Pr Nd Pm Sm Eu Gd Tb Dy Но Er Tm Yb 138.90547 140.116 140.90765 144.244 (145) 150.36 151.964 157.25 158.92535 162.500 164.93032 167.259 168.93421 173.04 Metalloid 89 90 91 92 93 94 95 96 97 98 99 100 101 102 Actinides Ac Th Pa Np Pu Am Cm Bk Es Fm Md No (227) 232.03806 231.03588 238.02891 (237) (244) (243) (247) (247) (251) (252) (257) (258) (259) Nonmetal Period
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
a If we look the group VA element of giv...View the full answer

Answered By

Aditya bhargaw
I am pursuing my bachelor's degree in chemistry and I am final year student and I am also work in a coaching institute where I teach High school students chemistry subject. It is best things to teach others and learn also for better results in the future
I will give my knowledge students as much as I can.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Suppose a platform tries to recommend the best movie ton Ann and Ben. Movie 1's quality is uniformly distributed from 0 ton 1. Movie 2's quality is uniformly distributed from 0.5 to 1. n Suppose the...
-
Refer to the periodic table and obtain the group and period for each of the following elements. Also determine whether the element is a metal, nonmetal, or metalloid. a. S b. Fe c. Ba d. Cu e. Ne
-
Refer to the table and answer the following questions : 1. Complete the table ? 2. If the period covered by table above is one year, the interest rate paid on bonds is . 3. The optimal average money...
-
Refer to the information in BE4-7 for Hébert Company. Prepare the correcting journal entries. Information in BE4-7 1. A collection of cash on account from a customer for $750 was recorded as a...
-
Why is it important to skim through the entire table of annotations before reading the annotations themselves?
-
An inexperienced bookkeeper prepared the following trial balance that does not balance. Prepare a correct trial balance, assuming all account balances are normal. LO9 BIRELLIE COMPANY Trial Balance...
-
Computing Unit Prices. Calculate the unit price of each of the following items.
-
Danish Hospital recently installed a RAP Scanner, which is a diagnostic tool used both in suspected cancer cases and for detecting certain birth defects while the fetus is still in the womb. The...
-
Food Incorporated, a public company following IFRS, has a machine that processes and packages tuna in oil. The machine originally cost $100,000 and is being amortized on a straight-line basis over 20...
-
The Google 10-K Form is reproduced online at www.wiley.com/college/pratt. REQUIRED: a. Review the Google SEC Form 10-K and analyze the financial statements by assessing Googles earning power and...
-
Refer to the periodic table (Figure 2.15 or inside front cover) and answer the following questions. a. What Group VIA element is a metalloid? b. What is the Group III A element in Period 3? Figure...
-
Write the systematic name for each of the following compounds represented by a molecular model. a. b. c. Se
-
1. Develop a social media plan for that business using what you learned in this chapter. 2. What are the objectives of your social media plan? 3. What social media tools would you choose and why? How...
-
4. (15pt) A group of students were asked if they have ever driven after drinking. They also were asked, "How many days per month do you drink at least two beers?" In the following discussion, 7 = the...
-
discuss how might you apply the concepts of Total Quality (TQ) to your personal and work environment. Consider your relations with others and your daily activities interactions with. Share the...
-
Dr. Bernstein wants to expand his radiology practice. Dr. Bernstein is researching various local banks for the best certificate of deposit rate to fund his expansion. One bank is willing to offer him...
-
An airplane is flying with a velocity of 240 m/s at an angle of 30.0 with the horizontal, as the drawing shows. When the altitude of the plane is 2.4 km, a flare is released from the plane. The flare...
-
Katsura Corporation incurred pre - operating costs: Investigatory expenses of $ 1 8 , 0 0 0 New employee training $ 2 5 , 0 0 0 Advertising $ 1 0 , 0 0 0 Land and building for use as a retail store...
-
Write a program that displays five texts vertically, as shown in Figure 14.44a. Set a random color and opacity for each text and set the font of each text to Times Roman, bold, italic, and 22 pixels....
-
Some people argue that the internal control requirements of the Sarbanes-Oxley Act (SOX) put U.S. companies at a competitive disadvantage to companies outside the United States. Discuss the...
-
Show that F [f1 (t) f2 (t)] = 1- 2/ F1 (x) F2 ( - x) dx
-
Find the Fourier transform of the function f (t) = e-a|t|.
-
Find the Fourier transform of the function f (t0 = 12e-2|t| cos 4t.
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
Brief Exercise 10-6 Flint Inc. purchased land, building, and equipment from Laguna Corporation for a cash payment of $327,600. The estimated fair values of the assets are land $62,400, building...
-
"faithful respresentation" is the overriding principle that should be followed in ones prepaparation of IFRS-based financial statement. what is it? explain it fully quoting IAS. how this this...

Study smarter with the SolutionInn App


