Suggest an efficient synthesis for the following transformation: En Br
Question:
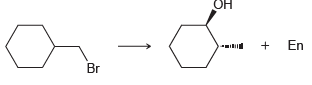
Transcribed Image Text:
Он En Br
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (18 reviews)
c Br 1 NaO...View the full answer

Answered By

Sagar Kumar
I am Mechanical Engineer with CGPA of 3.98 out of 4.00 from Pakistan. I went to Government Boys Degree College, Sehwan for high school studies.
I appeared in NUST Entrance Exam for admission in university and ranked #516. My mathematics are excellent and I have participated in many math competitions and also won many of them. Recently, I participated in International Youth Math Challenge and was awarded with Gold Honor. Now, I am also an ambassador at International Youth Math Challenge,
I have been teaching when I was in 9th class class year 2012. I have taught students from 6th class to university level.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Devise an efficient synthesis for the following transformation (recall that aldehydes are more reactive than ketones): H.
-
Propose an efficient synthesis for the following transformation. Br
-
Propose an efficient synthesis for the following transformation.
-
CASE STUDY. Case Study Chapters 1 and 2. Please post both case studies in Assignment Drop Box as one MS Word apa formate document. Note: See template provided for case study papers. Chapter 1 - Listo...
-
How does outside financing of a "very small" business, such as a bicycle repair shop, proceed?
-
Exercise 3.1.29 described a method of collecting urine samples that the researchers thought would be representative of climbers on Mont Blanc. Because the observations were taken from urinals, we can...
-
Describe the three risk monitoring tools that were discussed in this chapter. AppendixLO1
-
Presented below are selected accounts for Acevedo Company as reported in the worksheet at the end of May 2012. InstructionsComplete the worksheet by extending amounts reported in the adjusted trial...
-
What is the amount of gross accounts receivables and the allowance for doubtful accounts for both 2022 and 2021 ? What is a bit unusual about the relationship between these numbers between years?...
-
Marvel Studio's motion picture Guardians of the Galaxy opened over the first two days of the 2014 Labor Day weekend to a record-breaking $94.3 million in ticket sales revenue in North America (the...
-
The long-run downward trend in commodity prices is consistent with the idea that: a. We are quickly running out of resources. b. Resource demands have been increasing faster than resource supplies....
-
Propose a plausible mechanism for the following tautomerization process: . CH CH3 ,
-
Do you believe that licensing in represents a feasible long-term product development strategy for a company? Discuss in relation to in-house product development.
-
As the human resource manager, how would you evaluate the training needs of your staff? How can you ensure that the training you would provide is effective? What data might be used to make your...
-
MARYLAND CORPORATION manufactures three liquid products - Alpha, Beta and Gamma using a joint process with direct materials, direct labor and overhead totaling $560,000 per batch. In addition, the...
-
Three common organizational structures. Mention one organization for each organizational structure which is following a specific organizational structure. Also, provide support to your answer by...
-
You are a retail manager at Kitchen Nightmare, a relatively new store at the mall that sells mostly items for kitchens, like forks, oven mitts, etc.. You have been open since the fall of 2021 and...
-
Examine the extent to which the Department of Veteran Affairs has established any processes or procedures to ensure knowledge retention of departing employees. Why is it important to manage the...
-
Use the graph of y = f(x) to determine if f is one-to-one. Does f have an inverse? y 3 y = f(x) 3 X
-
(a) Given a mean free path = 0.4 nm and a mean speed vav = 1.17 105 m/s for the current flow in copper at a temperature of 300 K, calculate the classical value for the resistivity of copper. (b)...
-
How would you prepare o-hydroxyphenyl-acetaldehyde from phenol? More than one step is required. HO o-Hydroxyphenylacetaldehyde CH-CO
-
Imagine that you have treated (2R, 3R)-2, 3-epoxy-3-methylpentane with aqueous acid to carry out a ring-opening reaction. (a) Draw the epoxide, showing stereochemistry. (b) Draw and name the product,...
-
Identify the reagents a?c in the following scheme: CH C -CH CH H -CH H.
-
Indicate whether the following managerial policy increases the risk of a death spiral:Use of low operating leverage for productionGroup of answer choicesTrueFalse
-
It is typically inappropriate to include the costs of excess capacity in product prices; instead, it should be written off directly to an expense account.Group of answer choicesTrueFalse
-
Firms can avoid the death spiral by excluding excess capacity from their activity bases. Group of answer choicesTrueFalse

Study smarter with the SolutionInn App


