The following compound is optically inactive. Explain why. CH3 H3C-
Question:
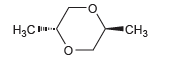
Transcribed Image Text:
"CH3 H3C- НаС в
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 54% (11 reviews)
This compound has a center of inver...View the full answer

Answered By

Anthony Ngatia
I have three academic degrees i.e bachelors degree in Education(English & Literature),bachelors degree in business administration(entrepreneurship option),and masters degree in business administration(strategic management) in addition to a diploma in business management.I have spent much of my life in the academia where I have taught at high school,middle level colleges level and at university level.I have been an active academic essays writer since 2011 where I have worked with some of the most reputable essay companies based in Europe and in the US.I have over the years perfected my academic writing skills as a result of tackling numerous different assignments.I do not plagiarize and I maintain competitive quality in all the assignments that I handle.I am driven by strong work ethics and a firm conviction that I should "Do Unto others as I would Like them to do to me".
4.80+
76+ Reviews
152+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Compound X is optically inactive and has the formula C16H16Br2. On treatment with strong base, X gives hydrocarbon Y, C16H14. Compound Y absorbs 2 equivalents of hydrogen when reduced over a...
-
A compound D with the molecular formula C6H12 is optically inactive but can be resolved into enantiomers. On catalytic hydrogenation, D is converted to E (C6H14) and E is optically inactive. Propose...
-
Compound F has the molecular formula C5H8 and is optically active. On catalytic hydrogenation F yields G (C5H12) and G is optically inactive. Propose structures for F and G.
-
Water flowing in a positive x-direction passes through a 90 elbow in a 6-inch-diameter pipeline and heads in a positive y-direction with a flow rate of 3.05 ft3/sec. Compute the magnitude and...
-
In a paragraph or two summarize the purpose of the article? Give the authors' definition of learning organizations. Critique this definition using scholarly support for every claim you make. What is...
-
In Exercises sketch the graph of '. Explain how you found your answer. 7 6 4 3 2 y + X 1 2 3 4 5 6 7 8
-
The most popular type of movie among 100,000 online movie rental subscribers
-
EZPAK Manufacturing produces filament packaging tape. In 2010, EZPAK Manufacturing produced and sold 15 million rolls of tape. The company has recently expanded its capacity, so it can now produce up...
-
quiz 8 q.6 Arnell Industries has just issued $25 million in debt (at par). The firm will pay interest only on this debt. Arnell's marginal tax rate is expected to be 21% for the foreseeable future....
-
Suppose that Aviva can earn supplemental in-come by working overtime. She intends to use any income she earns to buy shares of stock in a corporation, with the intention of leaving the shares to her...
-
How are the suns energy, prevailing winds, and surface-ocean currents related?
-
What is the El Nio-Southern Oscillation (ENSO)? What are some of its global effects?
-
An object whose mass is 400 kg is located at an elevation of 25 m above the surface of the earth. For g= 9.78/s2, determine the gravitational potential energy of the object, in kJ, relative to the...
-
Convert the following information into: a) a semantic net b) a frame-based representation A Ford is a type of car. Bob owns two cars. Bob parks his car at home.His house is in California, which is a...
-
Visit www.pearsonglobaleditions.com/malhotra to read the video case and view the accompanying video. Marriott: Marketing Research Leads to Expanded Offerings highlights Marriotts success in using...
-
The water level in a tank is \(20 \mathrm{~m}\) above the ground. A hose is connected to the bottom of the tank, and the nozzle at the end of the hose is pointed straight up. The tank cover is...
-
A simple experiment has long been used to demonstrate how negative pressure prevents water from being spilled out of an inverted glass. A glass that is fully filled by water and covered with a thin...
-
A golf ball is hit on a level fairway. When it lands, its velocity vector has rotated through an angle of 90. What was the launch angle of the golf ball? Pyo By Dyz =0 Uso Range R x max dya
-
In Exercises 5764, find the vertical asymptotes, if any, the horizontal asymptote, if one exists, and the slant asymptote, if there is one, of the graph of each rational function. Then graph the...
-
Suppose a population of bacteria doubles every hour, but that 1.0 x 106 individuals are removed before reproduction to be converted into valuable biological by-products. Suppose the population begins...
-
Arrange these alkenes in order of increasing rate of reactio0n with HCI: CH, CH2=CH2 a) CH;CH2CH=CH2 CH;CH,C=CH, -CH=CH; CH=CH2 CH,CH-CH b) CHO
-
Show the structure of the carbocations that are formed in the reaction of HBr with 2-hexena and explain why two products are formed.
-
Show the products of thesereactions: + HCI b) + HF + HI CH3 + HBr CH3 + HCI CH,CH, (p)
-
1. Determine the value of the right to use asset and lease liability at commencement of the lease.
-
Problem 22-1 The management of Sunland Instrument Company had concluded, with the concurrence of its independent auditors, that results of operations would be more fairly presented if Sunland changed...
-
Question 4. - Week 9. What are the major competitive issues General Electric faces when managing cooperative strategies? - (7 marks)

Study smarter with the SolutionInn App


