The following compounds cannot be made using only reactions that we learned in this chapter. For each
Question:
(a)
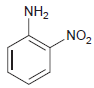
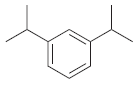
(b)
Transcribed Image Text:
NH2 „NO2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 73% (15 reviews)
a Nitration cannot be achieved effectively ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which of the following compounds cannot be prepared by an acetoacetic ester synthesis? Explain. (a) Phenyl acetone (b) Acetophenone (c) 3, 3-Dimethyl-2-butanone
-
Show how the following compounds could be prepared, using only the indicated starting material as the source of carbon: a. b. c. d. CH CH3CCH3 from CH3CHCH3 CH3 CH3CH-CCH3 O CH3 CH3C CHCH3 CH CH2CH
-
Show how the following compounds could be prepared, using only the indicated starting material as the source of carbon: a. b. c. d. e. f. OH HO
-
Simplify the expressions in Problems 3138. (3x - 1) (x + 3x - 2)
-
Which method of valuing a business is best? Why?
-
The height y (in m) of the Gateway Arch in St. Louis (see Fig. 13.5) is given by y = 230.9 19.5(e x/38.9 + e /38.9 ), where x is the distance (in m) from the point on the ground level directly below...
-
P 20-4 Governmental fund conversion worksheet The postclosing trial balance for the City of Fort Collins governmental funds at June 30, 2016, shows the following ledger account balances: DR CR Cash...
-
In the past, the rules of discovery were very restrictive, and trials often turned on elements of surprise. For example, a plaintiff would not necessarily know until the trial what the defendants...
-
ASAP ITS final Preble Company manufactures one product. Its variable manufacturing overhead is applied to production based on direct labour-hours and its standard cost card per unit is as follows:...
-
If electron-positron annihilation into fermion-antifermion pairs were only mediated by a virtual photon... more than one answer possible 1. the cross-section would be proportional to the square of...
-
Using only reactions that we learned in this chapter, there are two different ways to prepare the following compound from benzene. Identify both ways, and then determine which way is likely to...
-
Starting with benzene and using any other necessary reagents of your choice, design a synthesis for each of the following compounds. In some cases, there may be more than one plausible answer. (a)...
-
Solve the given differential equation by undetermined coefficients. y'' + 2y' + 2y = 5e 6x
-
As an official sponsor of the Olympics, what specific benefit did John Hancock use to help drive sales in their national offices?
-
assumes that Nia has both a discount rate of zero and faces an interest rate of zero. These assumptions made calculating her constant level of consumption expenditure of $56,000 fairly...
-
Paul Petersen lives in Northern California. He owns a BMW car worth about $20,000. He wants to take a trip to Nevada with his girlfriend Patricia, who lives in Los Angeles. He takes his car into...
-
Do you see gendered patterns of interaction in personal relationships? Does knowing about gender linked patterns affect how other interpret on what happens in a relationships?
-
Significance For bone density scores that are normally distributed with a mean of 0 and a standard deviation of 1, find the percentage of scores that are significantly high (or at least 2 standard...
-
Lilac Skin Care Company consists of two departments, Blending and Filling. The Filling Department received 45,000 ounces from the Blending Department. During the period, the Filling Department...
-
Ann hires a nanny to watch her two children while she works at a local hospital. She pays the 19 year-old nanny $125 per week for 48 weeks during the current year. a. What is the employers portion of...
-
Identify the following two isomeric alkyl halides (C5H11Br) from their 300-MHz NMR spectra, which are as follows: Compound A: 0.91 (6/7, d, J = 6 Hz); 1.7-1.8 (3H, complex); 3.42 (2H, t, J = 6 Hz)...
-
Explain how the NMR spectra of 1, 2, 2-trimethyl-1-propanol would change following a D20 shake.
-
How would the NMR spectrum of ethyl fluoride differ from that of ethyl chloride?
-
please help Problem 13-7 (Algo) Prepare a Statement of Cash Flows [LO13-1, LO13-2] [The following information applies to the questions displayed below.] Comparative financial statements for Weaver...
-
A firm has 1000 shareholders, each of whom own $59 in shares. The firm uses $28000 to repurchase shares. What percentage of the firm did each of the remaining shareholders own before the repurchase,...
-
Vancouver Bank agrees to lend $ 180,000 to Surrey Corp. on November 1, 2020 and the company signs a six-month, 6% note maturing on May 1, 2021. Surrey Corp. follows IFRS and has a December 31 fiscal...

Study smarter with the SolutionInn App


