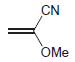
The following monomer can be polymerized under either acidic or basic conditions. Explain. CN OMe
Question:
Transcribed Image Text:
CN OMe
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (18 reviews)
It bears an electronwithdrawing group ...View the full answer

Answered By

Munir Ahmed Jakhro
I am professional Tutor of of Business Courses, I did my four years Bachelor Degree from one of the Top Business schools of World "Institute of Business Administration" in year 2013. Since then I have been working as Tutor of Accounting, Finance tutor on different online platforms like this website. I am have experience of 6 years teaching business courses to students online and offline my professional job at national savings also helped me in accounting understanding .
4.90+
8+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Although propylene can be polymerized with a free-radical initiator, the molecular weight of the polymer is never very high by this method because chain transfer from the methyl group of propylene...
-
Methyl methacrylate (Table 14.1) can be polymerized by catalytic amounts of n butyllithium at 78C. Using eqs. 14.17 and 14.18 as a model, write a mechanism for the reaction. Show how the intermediate...
-
Ethylene oxide can be polymerized by base to give carbowax, a water-soluble wax. Suggest a mechanism for the reaction. CH,-CH, HOCH OCH2CH2OH ethylene oxide
-
Verde Company produces wheels for bicycles. During the year, 657,000 wheels were produced. The actual labor used was 368,000 hours at $9.50 per hour. Verde has the following labor standards: 1)...
-
The Windshield Doctors repair chips in car windshields. The company incurred the following operating costs for the month of March 2018: Salaries and wages .................................. $ 12,000...
-
Many common titrations involve the reaction of an acid with a base. If 24.75 mL of 0.503 M NaOH solution is used to titrate a 15.00-mL sample of sulfuric acid, H 2 SO 4 , what is the concentration of...
-
Weekly Earnings A labor organization states that the median weekly earnings of male workers is greater than $798. To test this claim, you randomly select 70 male workers and ask each to provide his...
-
The following quarterly cost data have been accumulated for Oakeson Mfg. Inc: Raw materialsbeginning inventory (Jan. 1, 2011) . . . . . . . . . 90 units @ $7.00 Purchases . . . . . . . . . . . . . ....
-
Problem 8 - 2 1 ( Algo ) Schedules of Expected Cash Collections and Disbursements [ LO 8 - 2 , LO 8 - 4 , LO 8 - 8 ] You have been asked to prepare a December cash budget for Ashton Company, a...
-
True-Lens, Inc., is considering producing longwearing contact lenses. Fixed costs will be $24,000, with a variable cost per set of lenses of $8. The lenses will sell to optometrists for $24 per set....
-
Propose a mechanism for the acid-catalyzed hydrolysis of PET to regenerate the monomers terephthalic acid and ethylene glycol.
-
Polyethylene is used to make Ziploc bags and folding tables. Identify which of these applications is most likely to be made from HDPE and which is most likely to be made from LDPE.
-
A shot fired from a gun with a muzzle velocity of 1200 feet per second is to hit a target 3000 feet away. Determine the minimum angle of elevation of the gun.
-
Micro-Brush requires a new component for their laptop cleaning machines. The company must decide whether to make or buy them. If it decides to make them. Should it use process A or process B? Use a...
-
Moving from a fee-for-service to a managed care delivery system set up a series of expectations (page 421). How many of these expectations are realistic? How many have been realized?
-
2. A 55 kg human is shot out the end of a cannon with a speed of 18 m/s at an angle of 60. Ignore friction and solve this problem with energy conservation. As he exits the cannon, find: a. horizontal...
-
Theoretical Background: Information Assurance (IA) architecture also known as security architecture is about planning, integrating and continually monitoring the resources of an organization so they...
-
AZCN recommends Microsoft Lens or Adobe Scan; download one of these to yo phone via your phone's app store 2. Place the document you want to scan on a flat, well-lit surface. Make sure the document...
-
Evaluate the following iterated integrals. 2 [S 0 xy dx dy
-
Define the essential properties of the following types of operating systems: a. Batch b. Interactive c. Time sharing d. Real time e. Network f. Parallel g. Distributed h. Clustered i. Handheld
-
Ketones and aldehydes react with primary amines to give imines. They react with secondary amines to give enamines (vinyl amines). (a) For review, propose a mechanism for the following formation of an...
-
Rank each set of compounds in order of increasing basicity. (a) NaOH, NH3, CH3NH2, PhNH2 (b) Aniline, p-methylaniline, p-nitroaniline (c) Aniline, pyrrole, pyridine, piperidine (d) Pyrrole,...
-
The following partial IR spectra correspond to a primary amine, a secondary amine, and an alcohol. Give the functional group for each spectrum. 2.5 100 3 3.5 4 3 4 2.5 100 3.5 4 100 (by 3500 4000...
-
5. Which of the following is the cheapest for a borrower? a. 6.7% annual money market basis b. 6.7% semi-annual money market basis c. 6.7% annual bond basis d. 6.7% semi-annual bond basis.
-
Waterloo Industries pays 30 percent corporate income taxes, and its after-tax MARR is 24 percent. A project has a before-tax IRR of 26 percent. Should the project be approved? What would your...
-
Imagine you are an Investor in the Stock Market. Identify three companies in the Korean Stock Market (KOSPI) where you would like to invest. Explain your answer

Study smarter with the SolutionInn App


