What reagents would you use to perform each of the following transformations? a. b. H.
Question:
a.
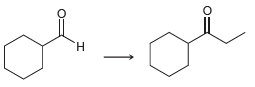
b.
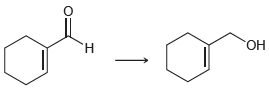
Transcribed Image Text:
H. н ОН
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 86% (15 reviews)
a b H 1 ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What reagents would you use to prepare the following compounds? a. b. CH CCH CH:C 0 CH3CCH-CH-CH (COCH-CH3 )2
-
What reagents would you use to prepare each of the following thiols: a. b. c. SH SH
-
What reagents would you use to bring about each step of the following syntheses? (a) (b) (c) (d) OH HiO4 (Section 22.6D) OH +NIN:
-
Assume a portfolio of two assets with the following total value (Al) covariance matrix 1 -0.5 -0.5 1 The sum of the 99% component VaRs of the two assets is:
-
Reids Company, which uses net present value to analyze investments, requires a 10% minimum rate of return. A staff assistant recently calculated a $500,000 machine's net present value to be $86,400,...
-
Find both first partial derivatives. z = xey
-
Remembering the target market segments you identified in Chapter 8 for your marketing plan:
-
Many organizations focus their strategy on providing high-quality customer service and consequently place metrics concerning customer relationship management on their balanced scorecards. Consider...
-
CALCULATOR FULL SCREEN PRINTER VERSION BACH NIK Question 12 Blue Spruce Company incurs the following expenditures in purchasing a truck cash price $31,000, accident insurance $3,500, sales taxes...
-
Triangle EFG is isosceles with EG = 9 cm. GH is an arc of a circle, centre F, with angle HFG = 0.6 radians, Find a. The area of sector of HFG b. The area of triangle EFG c. The area of the shaded...
-
A 1.25 mole sample of an ideal gas is expanded from 320. K and an initial pressure of 3.10 bar to a final pressure of 1.00 bar, and C P,m = 5/2R. Calculate w for the following two cases: a. The...
-
Propose a mechanism for the following reaction. NaH Br
-
List the public-good aspects (if any) of the following goods: safety, street names, and a steak dinner.
-
Use least square regression to fit a straight line to the following data taken from the conductance (S/m) of a material with respect to temperature (C) of a composite material used to absorb heat....
-
A pile group consists of nine friction piles in clay soil (see Figure 10-40). The diameter of each pile is 16 in., and the embedded length is 30 ft each. Center-to-center pile spacing is 4 ft. Soil...
-
The rigid bar EBC is supported by two links AB and CD as shown in Figure 1. The Link AB is made of aluminum (E = 70 GPa) and the link CD is made of steel (E = 200 GPa). Both links have a Width = 30...
-
a well-insulated storage tank was pressurized under ideal gas conditions by air flowing into the tank. We used the first law to estimate the final temperature of the gas in the tank, Tf,tank- = We...
-
Transportation of natural gas is commonly done via pipelinesacross long distances. A company uses a 0.6-m diameter pipe totransport natural gas. Then pumping stations are located atdifferent points...
-
In Problems 18, find the real solution(s), if any, of each equation. |x2| = 1
-
Charles owns an office building and land that are used in his trade or business. The office building and land were acquired in 1978 for $800,000 and $100,000, respectively. During the current year,...
-
Predict the product of the reaction of valine with the following reagents: (a) CH3CH2OH, acid (b) Di-tert-butyl dicarbonate (c) KOH, H2O (d) CH3COCl, pyridine then H2O
-
Show how you could use the acetamidomalonate method to prepare the following amino acids: (a) Leucine (b) Tryptophan
-
Show how you could prepare the following amino acids using a reductive amination: (a) Methionine (b) Isoleucine
-
Randy (48) takes a $22,000 distribution from his IRA to pay some of his daughter's $28,000 qualified education expenses at an eligible educational institution. His daughter paid $18,000 of her...
-
The takeover specialist chose to use the value derived from dividend discount model, while the directors prefer to use Net Realisable Value approach. Critically discuss the reasoning of each parties...
-
Tony and Suzie graduate from college in May 2021 and begin developing their new business. They begin by offering clinics for basic outdoor activities such as mountain biking or kayaking. Upon...

Study smarter with the SolutionInn App


