Question: When 3,3-dichloropentane is treated with excess sodium amide in liquid ammonia, the initial product is 2-pentyne: However, under these conditions, this internal alkyne quickly isomerizes
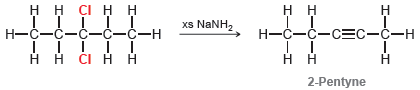
However, under these conditions, this internal alkyne quickly isomerizes to form a terminal alkyne that is subsequently deprotonated to form an alkynide ion:
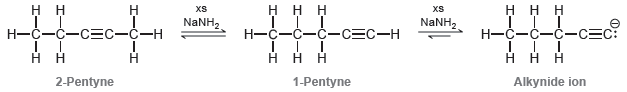
The isomerization process is believed to occur via a mechanism with the following four steps:
(1) Deprotonate
(2) Protonate
(3) Deprotonate
(4) Protonate.
Using these four steps as a guide, try to draw the mechanism for isomerization using resonance structures whenever possible. Explain why the equilibrium favors formation of the terminal alkyne.
CI xs NANH2 ---- CI 2-Pentyne IICIH XS NaNH2 XS NaNH, - ---- Alkynide ion 1-Pentyne 2-Pentyne
Step by Step Solution
3.41 Rating (167 Votes )
There are 3 Steps involved in it
Hccccc 2pentyne ILL I 1pentyne NH ... View full answer

Get step-by-step solutions from verified subject matter experts


