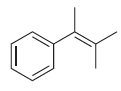
When 3-methyl-3-phenyl-1-butanamine is treated with sodium nitrite and HCl, a mixture of products is obtained. The following
Question:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 77% (9 reviews)
Na H H 10H H H...View the full answer

Answered By

Simon kingori
I am a tier-one market researcher and content developer who has been in this field for the last six years. I’ve run the freelancing gamut; from market research, data mining and SEO/SMM to copywriting, Content Development, you name it, I’ve done it. I’m extremely motivated, organized and disciplined – you have to be to work from home. My experience in Freelancing is invaluable- but what makes me a cut above the rest is my passion to deliver quality results to all my clients- it’s important to note, I've never had a dissatisfied client. Backed by a Masters degree in Computer Science from MOI university, I have the required skill set and burning passion and desire to deliver the best results for my clients. This is the reason why I am a cut above the rest. Having taken a Bsc. in computer science and statistics, I deal with all round fields in the IT category. It is a field i enjoy working in as it is dynamic and new things present themselves every day for research and exploration.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
(a) Give the structures or build molecular models and provide an acceptable name for all the isomers of molecular formula C7H9N that contain a benzene ring. (b) Which one of these isomers is the...
-
(a) Give the structures or build molecular models and provide an acceptable name for all the isomers of molecular formula C7H9N that contain a benzene ring. (b) Which one of these isomers is the...
-
Each of the following reactions has been reported in the chemical literature and gives a single organic product in high yield. Identify the product in each case. (a) 3-Benzyl-2, 6-dimethylphenol...
-
Popsters just opened a branch in Baguio City this December 2015. Summary of transactions for the first month of operations follows: 1. Baguio Branch received P15,750 cash from home office to start...
-
Find the probability for the experiment of drawing a card at random from a standard deck of 52 playing cards. 1. The card is a face card. 2. The card is not a face card. 3. The card is a red face...
-
In Exercises 93102, solve each equation. 52x.54x 125
-
A company reports net income of $75,000. Its weightedaverage common shares outstanding is 19,000. It has no other stock outstanding. Its earnings per share is: a. $4.69 b. $3.95 c. $3.75 d. $2.08 e....
-
1. A patent increases the incentive to develop new products because it ________ the price of the product and thus generates profit to cover a firms costs of _______. 2. In some cases, a patent is...
-
( a ) The following statements of financial position relate to Sefula and GOL at 3 1 December 2 0 2 3 : \ table [ [ Non - current assets, \ table [ [ Sefula ] , [ K ' 0 0 0
-
Discuss the advantages and disadvantages of guaranteeing reliable transfer of data between modules in the STREAMS abstraction.
-
Zippo lighters have been around for more than 80 years. But as the number of smokers in the United States continues to decline, Zippo has spent the last half century scouting the world for new...
-
Propose a synthesis for the following transformation: 'N'
-
Mutarotation causes the conversion of b-d-mannopyranose to -d-mannopyranose. Using Haworth projections, draw the equilibrium between the two pyranose forms and the open-chain form of d-mannose.
-
Pacifico Company, a U . S . - based importer of beer and wine, purchased 1 , 7 0 0 cases of Oktoberfest - style beer from a German supplier for 4 5 9 , 0 0 0 euros. Relevant U . S . dollar exchange...
-
Consider each of the following scenarios and identify a behavioral intervention to address each issue in family work. A teenager not complying with curfew. One member of the couple not picking up...
-
Sandy Crane Hospital expanded its maternity ward to add patient rooms for extended hospital stays. They negotiated a 15-year loan with monthly payments and a large sum of $250,000 due at the end of...
-
2 (39 marks) R QUESTION 2 (39 marks) Roundworm Ltd is a group of companies with a 31 December year-end. The Roundworm group financial statements for the years 20.21 and 20.22 are given below:...
-
Vino Veritas Company, a U.S.-based importer of wines and spirits, placed an order with a French supplier for 1,400 cases of wine at a price of 240 euros per case. The total purchase price is 336,000...
-
The function f(x) = 2.9x + 20.1 models the median height, f(x), in inches, of boys who are x months of age. The graph of f is shown. a. Describe how the graph can be obtained using transformations of...
-
By referring to Figure 13.18, determine the mass of each of the following salts required to form a saturated solution in 250 g of water at 30 oC: (a) KClO3, (b) Pb(NO3)2, (c) Ce2(SO4)3.
-
Draw the two chair conformations of each compound and label the substituents as axial and equatorial. In each case, determine which conformation is more stable. (a) Cis-1-ethyl-2-isopropylcyclohexane...
-
Using what you know about the conformational energetics of substituted cyclohexanes, predict which of the two decalin isomers is more stable. Explain your reasoning.
-
Convert each Newman projection to the equivalent line-angle formula, and assign the IUPAC name. (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) CH2CH3 CH2CH3 CH3 CH CH3 Br CH3 CH2CH3 CH2CHs Cl CH CH(CH32...
-
please help Problem 13-7 (Algo) Prepare a Statement of Cash Flows [LO13-1, LO13-2] [The following information applies to the questions displayed below.] Comparative financial statements for Weaver...
-
A firm has 1000 shareholders, each of whom own $59 in shares. The firm uses $28000 to repurchase shares. What percentage of the firm did each of the remaining shareholders own before the repurchase,...
-
Vancouver Bank agrees to lend $ 180,000 to Surrey Corp. on November 1, 2020 and the company signs a six-month, 6% note maturing on May 1, 2021. Surrey Corp. follows IFRS and has a December 31 fiscal...

Study smarter with the SolutionInn App


