When the following chiral epoxide is treated with aqueous sodium hydroxide, only one product is obtained, and
Question:
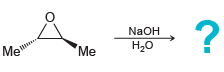
Transcribed Image Text:
NaOH Me H20 "Me
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)
The reaction yields a meso co...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict the major product obtained when each of the following aldehydes is treated with aqueous sodium hydroxide: (a) (b) (c) (d) H. H.
-
Draw all four -hydroxyaldehydes that are formed when a mixture of acetaldehyde and pentanal is treated with aqueous sodium hydroxide.
-
When treated with aqueous sodium hydroxide, 2,2-dimethylbutanal does not undergo an aldol addition reaction. Explain this observation.
-
Nu-Look Design, Inc., operated as a residential home improvement company. During calendar years 1996, 1997, and 1998, Ronald A. Stark not only was Nu-Looks sole shareholder and president but also...
-
Rock Inc. has three division, Granite, Lime and Nina. All fixed costs are unavoldable Following is the income statement for the previous year: a. What would Rock's profit margin be if the Lime...
-
What measures can be taken to protect your system from vulnerabilities like Eternal Blue? What should you do if you suspect your system is vulnerable to Eternal Blue?
-
Organization. The organization must be designed to put people in close contact with their suppliers and customers, internal or external. One way is to organize into customer-, product-, or...
-
Process Costing and Job-Order Costing Which method of determining product costs, job-order costing or process costing, would be more appropriate in each of the following situations? a. An Elmers glue...
-
How well is Costco performing from a financial perspective? Do some number-crunching using the data in case Exhibit 1 to support your answer. Use the financial ratios presented in Table 4.1 of...
-
Use technology to solve the following problem: According to a recent study, the weight of male babies less than two months old in the United States is normally distributed with mean 11.4 pounds and...
-
For a gas at a given temperature, the compression factor z is described by the empirical equation where P° = 1 bar. Calculate the fugacity coefficient for P = 150., 250., 350., 450., and 550....
-
When meso-2, 3-epoxybutane is treated with aqueous sodium hydroxide, two products are obtained. Draw both products and describe their relationship.
-
Import the salary data for the Baltimore Ravens NFL team from the website http://www.sportscity.com/nfl/salaries/baltimore-ravens-salaries/. Can you import data from other NFL teams from similar...
-
Consider the expression timing is everything in relation to the building of the TOMS brand. Besides the influence of recovering economic conditions and the increased affluence of potential customers,...
-
What is corporate strategy and why is it important? Choose a company with which you are familiar, and evaluate its corporate strategy, especially in regards to financial strategies. What are some...
-
Assignment Tasks: Review the following situations and for each pay period determine the employee's net pay by calculating what earnings & benefits are subject to Income Tax, Canada / Quebec Pension...
-
sample letter for signature change on bank accounts for principals of school
-
Use Excelshowing all work and formulasto complete the following: Prepare a flexible budget. Compute the sales volume variance and the variable cost volume variances based on a comparison between...
-
Write an equation of the line passing through the given point and satisfying the given condition. 5 (-3,-2); slope 0
-
The nitrogen atoms in N2 participate in multiple bonding, whereas those in hydrazine, N2H4, do not. (a) Draw Lewis structures for both molecules. (b) What is the hybridization of the nitrogen atoms...
-
Show the structure of the polymer that result from heating the following di-epoxide anddiamine: Heat + H2N- -NH2
-
Nomex, a polyamide used in such applications as fire-retardant clothing, is prepared by reaction of 1, 3-benzenediamine with 1, 3-benzenedicarbonyl chloride. Show the structure of Nomex.
-
Nylon 10, 10 is an extremely tough, strong polymer used to make reinforcing rods for concrete. Draw a segment of nylon 10, 10, and show its monomer units.
-
A stock is expected to pay a dividend of $1.50 at the end of the year (i.e., D 1 = $1.50), and it should continue to grow at a constant rate of 10% a year. If its required return is 14%, what is the...
-
The Hobby Shop has a checking account with a ledger balance of $1,700. The firm has $2,400 in uncollected deposits and $4,200 in outstanding checks. What is the amount of the disbursement float on...
-
An investment will pay you $34,000 in 11 years. If the appropriate discount rate is 6.1 percent compounded daily, what is the present value? (Use 365 days a year. Do not round intermediate...

Study smarter with the SolutionInn App


