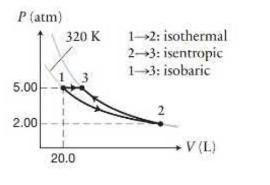
A sample of diatomic ideal gas ((gamma=1.4)) initially has a volume of (20.0 mathrm{~L}) and is at
Question:
A sample of diatomic ideal gas \((\gamma=1.4)\) initially has a volume of \(20.0 \mathrm{~L}\) and is at \(5.00 \mathrm{~atm}\) and \(320 \mathrm{~K}\). The gas undergoes the process shown in Figure P20.58. First it expands isothermally \((1 \rightarrow 2)\) until the pressure is \(2.00 \mathrm{~atm}\), and then it is compressed isentropically \((2 \rightarrow 3)\) until the pressure is \(5.00 \mathrm{~atm}\) again.
(a) Determine the volume of the gas after the isothermal expansion and the work done by the gas during the expansion.
(b) Determine the volume and temperature of the gas after the isentropic compression, and the work done on the gas during the compression.
(c) Determine the change in entropy of the gas by determining \(\Delta S\) for \(1 \rightarrow 2\) plus \(\Delta S\) for \(2 \rightarrow 3\).
(d) Show that the entropy change is the same when the gas undergoes the isobaric process \(1 \rightarrow 3. 00\)
Data from Figure P20.58
Step by Step Answer:






