An ideal monatomic gas expands isentropically from an initial pressure (P_{mathrm{i}}) and initial volume (V_{mathrm{i}}) to a
Question:
An ideal monatomic gas expands isentropically from an initial pressure \(P_{\mathrm{i}}\) and initial volume \(V_{\mathrm{i}}\) to a final pressure \(P_{\mathrm{t}}=P_{\mathrm{i}} / 20\) and final volume \(V_{\mathrm{t}}=6 V_{\mathrm{i}}\). What is the work done by the gas, expressed in terms of \(P_{\mathrm{i}}\) and \(V_{1}\) ? [Use Eq. 20. 8 (but remember that you want work done by the gas, so watch your signs) and the fact that \(P V^{\gamma}=\) constant for an isentropic expansion (Eq. 20. 46), with \(\gamma=\frac{5}{3}\) for a monatomic ideal gas.]
Data from Eq. 20. 8
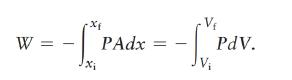
Data from Eq. 20.46
![]()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





