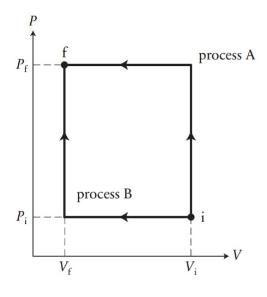
In Figure 20.28, an ideal gas is brought from an initial state (mathrm{i}) to a final state
Question:
In Figure 20.28, an ideal gas is brought from an initial state \(\mathrm{i}\) to a final state \(\mathrm{f}\) by two processes. The initial volume and pressure are \(0.50 \mathrm{~m}^{3}\) and \(100 \mathrm{kPa}\), and the final values for these variables are \(0.10 \mathrm{~m}^{3}\) and \(500 \mathrm{kPa}\). How much energy is transferred thermally to the gas during \((a)\) process \(\mathrm{A}\) and \((b)\) process B?
Data from Figure 20.28
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





