Question: When a certain ideal gas thermometer is placed in water at the freezing point, the mercury level in the right arm is (869 mathrm{~mm}) above
When a certain ideal gas thermometer is placed in water at the freezing point, the mercury level in the right arm is \(869 \mathrm{~mm}\) above the reference mark. How far is the mercury level above the reference mark when this thermometer is placed at room temperature where the atmospheric pressure is \(1.00 \mathrm{~atm}\) (See Table 20.1 for temperature values.)?
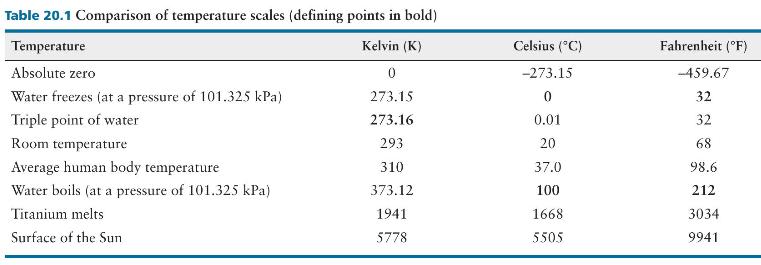
Table 20.1 Comparison of temperature scales (defining points in bold) Temperature Kelvin (K) Celsius (C) Fahrenheit (F) Absolute zero 0 -273.15 Water freezes (at a pressure of 101.325 kPa) 273.15 0 -459.67 32 Triple point of water 273.16 0.01 32 Room temperature 293 20 68 Average human body temperature 310 37.0 98.6 Water boils (at a pressure of 101.325 kPa) 373.12 100 212 Titanium melts 1941 1668 3034 Surface of the Sun 5778 5.505 9941
Step by Step Solution
3.32 Rating (149 Votes )
There are 3 Steps involved in it
Solutions Step 1 ANSWER Given The triple point of water is T 1 2... View full answer

Get step-by-step solutions from verified subject matter experts


