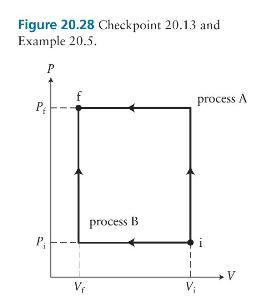
Which quasistatic process from the initial state (i) to the final state (f) in Figure 20.28 requires
Question:
Which quasistatic process from the initial state \(i\) to the final state \(f\) in Figure 20.28 requires more work done on the gas?
Transcribed Image Text:
Figure 20.28 Checkpoint 20.13 and Example 20.5. P P P process B V V process A V
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
Process A The vertical legs for both A and B are at con...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Physics questions
-
(a) For which of the two quasistatic processes shown in Figure 20.22, if either, is the change in entropy greater? (b) Is the energy transferred thermally to the gas the same along both paths? Figure...
-
In quasistatic process A of Figure 20.24, an ideal gas is brought from an initial to a final state while its temperature is held fixed. In quasistatic process B, an identical gas is brought in two...
-
The gas mixture from Problem 12.6 is compressed in a reversible adiabatic process from the initial state in the sample cylinder to a volume of 0.2 L. Determine the final temperature of the mixture...
-
I keep getting the second question wrong. Can you help me to getthat one, please thank you.I tried 8.66, and 8.67 does not are the correct answer A firm has 10 million shares outstanding with a...
-
Bethany incurred $20,000 in research and experimental costs for developing a specialized product during July of year 1.Bethany went through a lot of trouble and spent $10,000 in legal fees to receive...
-
You are considering three stocksA, B, and Cfor possible inclusion in your investment portfolio. Stock A has a beta of 0.80, stock B has a beta of 1.40, and stock C has a beta of -0.30. a. Rank these...
-
Use your results from Exercise 35 to construct a bar graph that shows the percentages of U.S. adults ages 25 and over based on employment status. Each category of employment status will have four...
-
Many small boats are made of fiberglass, which is derived from crude oil. Suppose that the price of oil rises. a. Using diagrams show what happens to the cost curves of an individual boat-making firm...
-
GOODWILL IMPAIRMENT: QUESTION: On May 17, 2012, Warren Buffett's Berkshire Hathaway announced an offer to buy 63 newspapers from Media General Inc. for $142 million in cash and provide debt financing...
-
Consider the three processes shown in Figure 20.29. Process B is isothermal. (a) For which of the three processes is the energy transferred thermally greatest? (b) For which of the three processes is...
-
What is the thermal energy associated with a gas sample that contains \(N\) hydrogen molecules at temperature \(T=300 \mathrm{~K}\) ?
-
Discuss the role of national and international standards in certifying vendors.
-
The relevance ( Relevance ) and the reliability ( Reliability ) represent two characters Key qualitative statistics of information n accountant. What What do these two mean? terms in an accounting...
-
A virtual memory system has a page size of 1024 bytes, six virtual pages, and five physical page frames. The page table is shown in Table Q2(d) as follows: Virtual Page Number (VPN) 0 Page Frame...
-
31. z = x + 2xy, determine which of (I)-(II) in Figure 12.31 are cross- sections with x fixed and which are cross-sections with y fixed. (1) (11) -2 -2+
-
WHAT DOES SOCIETY EXPECT FROM ORGANIZATIONS AND MANAGERS? Introduction: TOMS Shoes has a unique idea to promote corporate social responsibility. For each pair of shoes it sells, it donates a pair to...
-
1. A car heading east turns right at a corner. The car turns at a constant speed of 20.0 m/s. After 12 s, the car completes the turn, so that it is heading due south at 20.0 m/s. Calculate the car's...
-
Discuss some analytical or graphical approaches that organizations can use for analyzing performance data based on your experience and previous coursework.
-
The graph of an equation is given. (a) Find the intercepts. (b) Indicate whether the graph is symmetric with respect to the x-axis, the y-axis, or the origin. -3 6 -6 3 x
-
Indicate whether the following managerial policy increases the risk of a death spiral:Use of low operating leverage for productionGroup of answer choicesTrueFalse
-
It is typically inappropriate to include the costs of excess capacity in product prices; instead, it should be written off directly to an expense account.Group of answer choicesTrueFalse
-
Firms can avoid the death spiral by excluding excess capacity from their activity bases. Group of answer choicesTrueFalse

Study smarter with the SolutionInn App


