Question: (a) Derive a general relation for (E/p) T,n for electrochemical cells employing reactants in any state of matter. (b) E. Cohen and K. Piepenbroek (Z.
(a) Derive a general relation for (∂E/∂p)T,n for electrochemical cells employing reactants in any state of matter.
(b) E. Cohen and K. Piepenbroek (Z. Physik. Chem. 167A, 365 (1933)) calculated the change in volume for the reaction TlCl(s) + CNS−(aq) → TlCNS(s) + Cl−(aq) at 30°C from density data and obtained ∆rV = −2.666 ± 0.080 cm3 mol−1. They also measured the emf of the cell Tl(Hg)|TlCNS(s)|KCNS KCl|TlCl|Tl(Hg) at pressures up to 1500 atm. Their results are given in the following table:
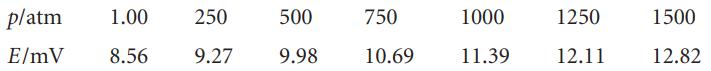
From this information, obtain (∂E/∂p)T,n at 30°C and compare to the value obtained from ∆rV.
(c) Fit the data to a polynomial for E against p. How constant is (∂E/∂p)T,n?
(d) From the polynomial, estimate an effective isothermal compressibility for the cell as a whole.
p/atm 1.00 250 E/mV 8.56 9.27 500 9.98 750 10.69 1000 11.39 1250 12.11 1500 12.82
Step by Step Solution
3.49 Rating (162 Votes )
There are 3 Steps involved in it
a The general relation for EpTn for electrochemical cells employing reactants in any state of matter ... View full answer

Get step-by-step solutions from verified subject matter experts


