A surface displaying a contour of the total charge density in LiH is shown here. The molecular
Question:
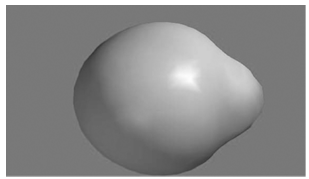
In problem 23.13
Images of molecular orbitals for LiH calculated using the minimal basis set are shown here. In these images, the smaller atom is H. The H1s AO has a lower energy than the Li2s AO. The energy of the MOs is (left to right) €“63.9 eV,€“7.92 eV, and +2.14 eV. Make a molecular orbital diagram for this molecule, associate the MOs with the images, and designate the MOs in the images as filled or empty. Which MO is the HOMO? Which MO is the LUMO? Do you expect the dipole moment in this molecule to have the negative end on H or Li?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





