(a) Two (unnormalized) excited state wavefunctions of the H atom are Normalize both functions to 1. (b)...
Question:
(a) Two (unnormalized) excited state wavefunctions of the H atom are
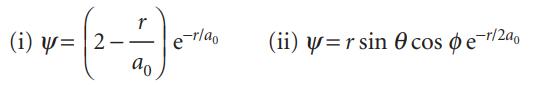
Normalize both functions to 1.
(b) Confirm that these two functions are mutually orthogonal.
Transcribed Image Text:
(i) u = 2 ਹੈ % -r/ao (ii) y=r sin cos e-r/2a0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What are the units of the H atom total energy eigenfunctions? Why is a 0 3/2 R(r) graphed in Figure 20.6 rather than R(r)?
-
A hydrogen atom is excited from its ground state to the state with n = 4. (a) How much energy must be absorbed by the atom? Consider the photon energies that can be emitted by the atom as it...
-
Two unnormalized functions of the hydrogen atoms are (2)2 1 1 2 2 sincos 2 Where N1 and N2 are the normalization constants The volume element for spherical polar coordinate is 2 sin and the limits...
-
Neutrons can be used in diffraction experiments to probe the lattice structure of crystalline solids. Since the neutron's wavelength needs to be on the order of the spacing between atoms in the...
-
You are given two paired samples with the following information: Based on these paired samples, test at the = 0.05 level whether the true median paired difference is 0. 2 le 3 4 O 2 1 5 9 438...
-
Graph each polynomial function. Factor first if the polynomial is not in factored form. (x) = (3x - 1)(x + 2) 2
-
a. Using the activity data in Exercise 27-19 prepare a cost of quality report. Assume that sales are $2,000,000. Round percentages to one decimal place. b. Using the activity data in Exercise prepare...
-
Various Time Value Situations Answer each of these unrelated questions. (a) On January 1, 2008, Aaron Brown Corporation sold a building that cost $250,000 and that had accumulated depreciation of...
-
4. 4: The Cost of Capital: Cost of Retained Earnings The cost of common equity is based on the rate of return that investors require on the company's common stock. New common equity is raised in two...
-
The accounting records of Nikolas Delivery Service show the following assets and liabilities as of the end of 2021 and 2022: The owner bought land for his equipment for $650,000. The business paid...
-
Determine which of the following functions are eigenfunctions of the inversion operator (which has the effect of making the replacement x x): (a) X 3 kx, (b) Cos kx, (c) X 2 + 3x 1. State the...
-
Calculate the average linear momentum of a particle described by the following wavefunctions: (a) E ikx , (b) Cos kx, (c) E x2 , where in each one x ranges from to +.
-
Determine the minimum number of workers needed and a schedule for tire following staffing requirements, giving workers two consecutive days off per week. Mon Tue Wed Thu Fri Sun Day Stoff needed 3 24...
-
Consider the situation you addressed in Problem and Exercise 3. Create numeric cost estimates for each of the costs you listed. Calculate the net present value and return on investment. Include a...
-
The output power \(\dot{W}\) of a spinning shaft is a function of torque \(T\) and angular velocity \(\omega\). Use dimensional analysis to express the relationship between \(\dot{W}, T\), and...
-
In groups of three, pick a local healthcare organization with which you are familiar. Conduct a SWOT analysis on the organization. After completing the SWOT analysis, use the template in exhibit 8.12...
-
The Dean Door Corporation (DDC) manufactures steel and aluminum exterior doors for commercial and residential applications. DDC landed a major contract as a supplier to Walker Homes, a builder of...
-
In Exercises 47 and 48, write a two-column proof. GIVENmWYZ = m/TWZ = 45 PROVE SWZ = ZXYW SW X Y N T
-
Consider the following statement: If the fuel cell had any value, it would have been fully developed by now and there would be many cars on the road that are powered by fuel cells. Do you agree or...
-
Select a mass spectrometric technique with the highest mass resolution for identifying an unknown compound being eluted from a liquid chromatography column
-
Verify that the radius ratio for (a) sixfold coordination is 0.414, and (b) for eightfold coordination is 0.732.
-
In an X-ray investigation, the following structure factors were determined (with F h00 =F h00 ): Construct the electron density along the corresponding direction. h F1,00 0 10 1 -10 2 8 3 -8 4 6 5 -6...
-
What are the values of the angle of the first three diffraction lines of bcc iron (atomic radius 126pm) when the X-ray wavelength is 72pm?
-
Pottery Ranch Inc. has been manufacturing its own finials for its curtain rods. The company is currently operating at 100% of capacity, and variable manufacturing overhead is charged to production at...
-
3. How much life insurance do you need? Calculating resources - Part 2 Aa Aa E Paolo and Maria Rossi have completed Step 1 of their needs analysis worksheet and determined that they need $2,323,000...
-
On March 1, LGE asks to extend its past-due $1,200 account payable to Tyson, Tyson agrees to accept $200 cash and a 180-day, 8%, $1,000 note payable to replace the account payable. (Use 360 days a...

Study smarter with the SolutionInn App


