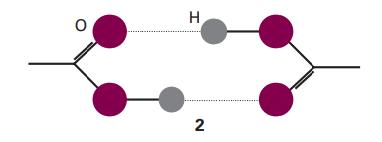
Acetic acid vapour contains a proportion of planar, hydrogen bonded dimers (2). The apparent dipole moment of
Question:
Acetic acid vapour contains a proportion of planar, hydrogen bonded dimers (2). The apparent dipole moment of molecules in pure gaseous acetic acid has a magnitude that increases with increasing temperature. Suggest an interpretation of this observation.
Transcribed Image Text:
H 2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
Explanation The dipole moment of a molecule is a measure of the electric charge distribution ...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula
Question Posted:
Students also viewed these Sciences questions
-
Acetic acid vapour contains a proportion of planar, hydrogen-bonded dimers. The relative permittivity of pure liquid acetic acid is 7.14 at 290K and increases with increasing temperature. Suggest an...
-
Gaseous acetic acid molecules have a certain tendency to form dimers. (A dimer is a molecule formed by the association of two identical, simpler molecules.) The equilibrium constant Kc at 25oC for...
-
Gaseous acetic acid molecules have a certain tendency to form dimers. (A dimer is a molecule formed by the association of two identical, simpler molecules.) The equilibrium constant Kp at 25oC for...
-
1 30 2 3 4 If A= = -1 2 1,B= 1 23 0 02 -1 1 2 5 9 13 (a) -1 2 4 -12 4 1 24 (C) -1 2 4 -2 24 then AB= 5 (b) -1 9 13 24 -2 2 4 (d) None of these
-
The flowchart for Problem 1 illustrates two alternative inventory ordering methods. Required a. Distinguish between a purchase requisition and a purchase order. b. Discuss the primary advantage of...
-
Brooklyn has been contributing to a traditional IRA for seven years (all deductible contributions) and has a total of $30,000 in the account. In 2020, she is 39 years old and has decided that she...
-
Describe how need for achievement, need for affiliation, and need for power relate to work motivation and performance. (L01)
-
In your audit of Aviary Industries for calendar year 2016, you found a number of matters that you believe represent possible adjustments to the company's books. These matters are described below....
-
The following data have been recorded for recently completed Job A34 on its job cost sheet. Direct materials cost was $1,000. A total of 100 direct labor-hours and 125 machine-hours were worked on...
-
The following information relates to La Greca Co. for the year 2022. Instructions After analyzing the data, prepare an income statement and a retained earnings statement for the year ending December...
-
Draw examples of the arrangements of electrical charges that correspond to monopoles, dipoles, quadrupoles, and octupoles. Suggest a reason for the different distance dependencies of their electric...
-
Plot the magnitude of the electric dipole moment of hydrogen peroxide as the HeOeOeH (azimuthal) angle changes from 0 to 2. Use the dimensions shown in 1. H 97 pm O 149 pm 1
-
Consider a 25-year, $350,000 mortgage with a rate of 7.25 percent. Ten years into the mortgage, rates have fallen to 5.4 percent. What would be the monthly saving to a home owner from refinancing the...
-
The following data are available for S&R company7 for its first month of operations: Direct materials Direct labor @P40/hr Job 101 Job 102 Job 103 P60,000 P90,000 P56,000 18,000 36,000 38,000...
-
1 2. Let A(x) = sin t + 1 dt, find A'(x) at x = 0, and 2 3. Evaluate the following definite integrals: 2 (a) (3x + 4x)dx 4 (b) xdx
-
A debt can be repaid with payments of $3912 today, $2436 in 2 years and $6770 in 5 years. What single payment will settle the debt 3 years from now if interest is 10.5% compounded quarterly?
-
With the PID/Freeze Frame/Snapshot data monitor function, input/output signal monitor items set in the start/stop control module can be selected and read out in real-time. Answer the following PID...
-
Which of the four global strategies (International, Multidomestic, Global-Standardization, or Transnational strategy) is 3M using? Is this the best strategy for it to use? Why or why not?
-
Find the derivative of the following functions. y = tan x + cot x
-
What mass of KBr (in grams) should you use to make 350.0 mL of a 1.30 M KBr solution?
-
Assume that a system has a very large number of energy levels given by the formula l = 0 l 2 with 0 = 1.75 10 -22 J, where l takes on the integral values 1, 2, 3, . . Assume further that the...
-
Determine in each of the following cases if the function in the first column is an eigenfunction of the operator in the second column. If so, what is the eigenvalue? a. x 2 ..x 2 / 8 d 2 /dx 2 b. x 3...
-
Is the function 2x 2 1 an eigenfunction of the operator (3/2 x 2 ) (d 2 /dx 2 ) + 2x (d /dx)? If so, what is the eigenvalue?
-
Al preparar el estado de resultados pro forma, cules de las siguientes partidas se deducen de las utilidades brutas para llegar a las ganancias despus de impuestos? Pregunta de seleccin mltiple....
-
Lawson Inc. is expanding its manufacturing plant, which requires an investment of $4 million in new equipment and plant modifications. Lawson's sales are expected to increase by $3 million per year...
-
20 On January 1, Year 1, X Company purchased equipment for $80,000. The company estimates that the equipment will have a useful life of 10 years and a residual value of $5,000. X Company depreciates...

Study smarter with the SolutionInn App


