An adult takes about 12 breaths per minute, inhaling roughly 500 mL of air with each breath.
Question:
An adult takes about 12 breaths per minute, inhaling roughly 500 mL of air with each breath. The molar compositions of the inspired and expired gases are as follows:
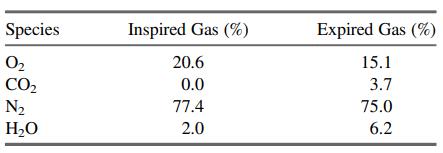
The inspired gas is at 24°C and 1 atm, and the expired gas is at body temperature and pressure (37°C and 1 atm). Nitrogen is not transported into or out of the blood in the lungs, so that (N2)in = (N2)out.
(a) Calculate the masses of O2, CO2, and H2O transferred from the pulmonary gases to the blood or vice versa (specify which) per minute.
(b) Calculate the volume of air exhaled per milliliter inhaled.
(c) At what rate (g/min) is this individual losing weight by merely breathing?
(d) The rate at which oxygen is transferred from the air in the lungs to the blood is roughly proportional to [(pO2)air - (pO2)blood] where (pO2)blood is a quantity related to the concentration of oxygen in the blood. Compared to regions where atmospheric pressure is 14.7 psia, what effect does the atmospheric pressure in Denver, which is approximately 12.1 psi, have on the transport rate and breathing rate? How does the body adjust to address this condition?
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





