Calculate the RedlichKwong parameters of fluorine from the values of the critical constants and compare your results
Question:
Table 7.4
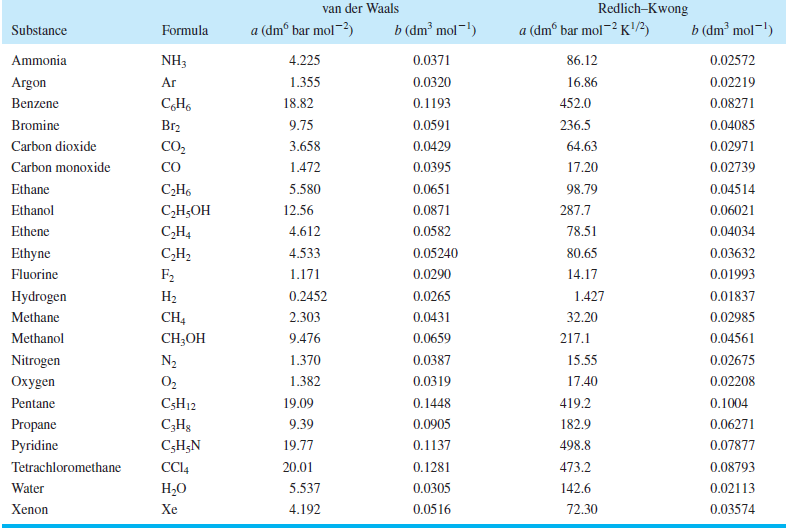
Transcribed Image Text:
Redlich–Kwong van der Waals b (dm² mol¬!) a (dmº bar mol-) b (dm² mol-!) a (dmº bar mol~² K) Substance Formula NH3 0.0371 Ammonia 4.225 86.12 0.02572 Argon Ar 1.355 0.0320 16.86 0.02219 452.0 Benzene 18.82 0.1193 0.08271 Br2 9.75 236.5 Bromine 0.0591 0.04085 CO2 0.0429 Carbon dioxide 3.658 64.63 0.02971 CO Carbon monoxide 1.472 0.0395 17.20 0.02739 0.04514 Ethane С-Н. 5.580 0.0651 98.79 Ethanol С-Н,ОН 12.56 0.0871 287.7 0.06021 Ethene C,H4 4.612 0.0582 78.51 0.04034 CH2 F2 0.05240 0.03632 Ethyne 4.533 80.65 Fluorine 1.171 0.0290 14.17 0.01993 1.427 Hydrogen На 0.2452 0.0265 0.01837 CH4 CH-OН 2.303 0.0431 Methane 32.20 0.02985 0.0659 Methanol 9.476 217.1 0.04561 Nitrogen 0.02675 N2 1.370 0.0387 15.55 0.0319 Охуgen O2 1.382 17.40 0.02208 Pentane C5H12 19.09 0.1448 419.2 0.1004 Propane C;H3 9.39 0.0905 182.9 0.06271 Pyridine C;H;N 19.77 0.1137 498.8 0.07877 Tetrachloromethane CC4 20.01 0.1281 473.2 0.08793 Water Н.О 5.537 0.0305 142.6 0.02113 4.192 Xenon Xe 0.0516 72.30 0.03574
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (14 reviews)
We use the values for the critical consta...View the full answer

Answered By

Saud Ur Rehman
Evaluating manufacturing processes by designing and conducting research programs; applying knowledge of product design, fabrication, assembly, tooling, and materials; conferring with equipment vendors; soliciting observations from operators. Developing manufacturing processes by studying product requirements; researching, designing, modifying, and testing manufacturing methods and equipment; conferring with equipment vendors. Keeping equipment operational by coordinating maintenance and repair services; following manufacturer's instructions and established procedures; requesting special service.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Related Video
In this video, A mixture of methanol and air in a large polycarbonate bottle is ignited. The resulting rapid combustion reaction, often accompanied by a dramatic ‘whoosh’ sound and flames, demonstrates the large amount of chemical energy released in the combustion of alcohol
Students also viewed these Sciences questions
-
Calculate the van der Waals parameters of carbon dioxide from the values of the critical constants and compare your results with the values for and b in Table 7.4. Table 7.4 RedlichKwong van der...
-
(a) 537 R (b) 3240 R. Compare your results with the KP values listed in Table A-28. TABLE A-28 Natural logarithms of the equilibrium constant K The equilibrium constant kp for the reaction AA+cC+D is...
-
(a) 298 K (b) 1800 K. Compare your results with the KP values listed in Table A-28. TABLE A-28 Natural logarithms of the equilibrium constant K The equilibrium constant kp for the reaction AA+cC+D is...
-
As a brand manager, for a product of your choice, develop a strategy for launching the product in a manner most likely to capture the attention of the product's primary target market. Write a...
-
Describe at least three things that your organization is doing to encourage ethical behavior and corporate social responsibility and at least three things that you think it should be doing to...
-
The price elasticity of demand for health care is often thought of as a measure of ex post moral hazard , which roughly captures the extent of overuse of medical care. Health insurance causes the...
-
A subsidiary has (1) a convertible preferred stock and (2) a convertible bond. How are these items factored into the computation of earnings per share for the business combination? LO4
-
On December 31, 2009, the Leslie Company held an investment in bonds of Kaufmann Company which it categorized as being held to maturity. At that time, the 8%, $100,000 face value bonds had a carrying...
-
Requirement: All learners will have to work on a course's report. The report is an academic write-up that details the whole report process undertaken by an undergraduate student, right from the...
-
Allie has bought a new apple orchard. The orchard has a single file of trees, numbered from 1 to N. Each tree has a certail number of ripe apples. Allie has a rule she wants to follow. She wants to...
-
Which is NOT a proper way to express the ratio of 10 out of each 100? a. 10 b. 0.10 c.10% d. 10/100
-
Have traditional patterns of consumption been radically changed by globalization?
-
Critical Thinking and Ethics Assume you are the CMO for an athletic shoe company. Over 75 percent of your sales comes from the United States and Europe. You have conducted business with Firm ABC in...
-
Q13. The probability that Ryan will roll a three using a standard die is 1/6. Let Y = number of times that Ryan has to roll a die in order to roll the first three. What is the expected value for Y?...
-
1. The following are data for two IT projects for a new database system. Prepare a spreadsheet for two projects, using the following data. Amounts are in thousands of dollars. Calculate the NPV for...
-
The Matsui Lubricants plant uses the weighted-average method to account for its work-in-process inventories. The accounting records show the following information for a particular day: Beginning WIP...
-
James Cook, a production department worker, is paid on hourly basis at a rate of $15 per hour. James works 40 hours per week. Any time James works over 40 hours, it is considered as overtime and he...
-
You just started working as a Health Service Manager within one of the following healthcare industries. First, choose an industry below to discuss the questions that follow: Ambulatory Surgery center...
-
In Problems 3340, write each interval as an inequality involving x, and graph each inequality on the real number line. (-, 2]
-
What is the amount of total interest dollars earned on a $5,000 deposit earning 6% for 20 years?
-
Prove the following fact about power series: If two power series in the same independent variable are equal to each other for all values of the independent variable, then any coefficient in one...
-
Find the interval of convergence for the series for cos (x).
-
Find the interval of convergence for the series for sin (x).
-
Create a Data Table to depict the future value when you vary the interest rate and the investment amount. Use the following assumptions: Interest Rates: Investment Amounts:-10.0% $10,000.00 -8.0%...
-
Isaac earns a base salary of $1250 per month and a graduated commission of 0.4% on the first $100,000 of sales, and 0.5% on sales over $100,000. Last month, Isaac's gross salary was $2025. What were...
-
Calculate the price, including both GST and PST, that an individual will pay for a car sold for $26,995.00 in Manitoba. (Assume GST = 5% and PST = 8%) a$29,154.60 b$30,234.40 c$30,504.35 d$28,334.75...

Study smarter with the SolutionInn App


