Describe what you would observe if you heated the liquid mixture at the composition corresponding to point
Question:
Figure 9.24b
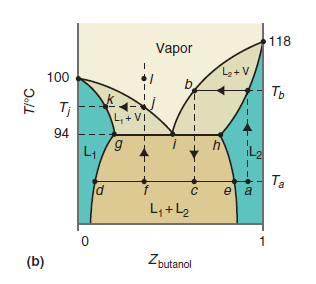
Transcribed Image Text:
118 Vapor 100 Ть Tj L + VI 94 6, L1 Ta e a L,+L2 Zbutanol (b) T/°C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
The mixture of two liquids will increase in temperature ...View the full answer

Answered By

Pramendra Kumar Singh
To be very honest, i have only a little experience regarding tutoring, that too was "applied" on my brother.
But regarding education, I am a confident man, just like John Wick. I obtained the highest possible CGPA in class 10th i.e 10CGPA. I was good at all the subjects, but my love for English, Social Studies and Mathematics were on a whole different level.
So for the above mentioned reason, i went for Mathematics for higher studies. Physics became my new love. And then, I ended up cracking JEE Mains, but couldn't go through Advanced. I gave a thought of taking a drop for a year, but my family situation was a bit shaky and a middle class boy couldnt afford to take the so-called "extreme risks".
Thus I gave a state level examination of Chhattisgarh state, where i live(btw). I obtained a rank of 274 out some lakhs (that was a relief back then). So, eventually i got admission into a good college (some say, topmost in our state).
But i, belonging to a class of kids who were brought up with 'being humble' as a centre of their principles, i worked hard. I still am. Electrical engineering, tough nut to crack. But Grind is the other name of Life. So i am still one of you students, working hard, having patience and also, trying to earn some money side-by-side to be free from always asking my parents for money. Believe me, after a certain age, u all will also feel the same way. In my case, i am slowly and steadily becoming allergic to asking for economic help, not atleast for my petrol and other daily expenses.
Also, you may find me using puns ans SARCASM a lot. Thats who i am. I joke a lot. I keep things simple. And, i am a man of 'wisdom' too. So i might employ some of my counselling skills with you too.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Describe what you would observe if you heated the solid at the composition 40. atomic percent Si in Figure 9.26 from 300.C to 1300.C.
-
A strip of aluminum metal is placed into a beaker containing 1.0 M CuSO4. (a) Will a spontaneous reaction occur? (b) If a spontaneous reaction does occur, write the half-reactions and describe what...
-
Describe what you would look for in a reaction involving gases in order to predict the sign of So. Explain.
-
On average, do people prefer a... 6 game package with 300-level, mid-court seats and a $20 gift certificate for $35/seat or a 3 game package with 300-level, corner seats that come with a hotdog and...
-
Jan finished culinary school and wants to start her own pastry business. She has few options, and believes that a sensitivity analysis would help her to make the best business decision. She is...
-
Orthogonally diagonalize the matrices in giving an orthogonal matrix P and a diagonal matrix D. To save you time, the eigenvalues in are the following: (17) 5, 5, 8; (18) 1, 2, 5; (19) 8, 1; (20) 3,...
-
Explain the advantages and disadvantages of franchising. LO.1
-
Frontera Companys output for the current period results in a $ 20,000 unfavorable direct labor rate variance and a $10,000 unfavorable direct labor efficiency variance. Production for the current...
-
Nicholas Inc. just paid a $2.00 dividend on its common stock and expects to continue growing dividends at an average rate of 4% each year, from now to infinity. If the required rate of return for...
-
The number of calories per candy bar for a random sample of standard-size candy bars is shown below. Estimate the mean number of calories per candy bar with 98% confidence. Assume that all variables...
-
Suppose the man does not run for 6 months over the winter due to snow on the ground. He resumes running once a week in the spring and records a running time = 12.97 minutes in his first week of...
-
The heat of fusion of water is 6.008 10 3 J mol 1 at its normal melting point of 273.15 K. Calculate the freezing point depression constant K f .
-
Time to be even more creative! Provide one null and one research hypothesis and an equation concerning each of the following: a. The amount of money spent on food by undergraduate studentathletes and...
-
a ) If F ( x ) = upper limit x and lower limit 8 dt , the F \' ( x ) = ? b ) If F ( x ) = upper limit 1 1 and lower limit x dt , the F \' ( x ) = ? c ) If F ( x ) = upper limit x ^ 6 and lower limit...
-
Please help me with this one can you do for me 1. the research on quantum dots. Find a resource that you think does the best job of explaining this. and provide short description of what you've...
-
During Year 1, Ashkar Company ordered a machine on January 1 at an invoice price of $28,000. On the date of delivery, January 2, the company paid $9,000 on the machine, with the balance on credit at...
-
An airplane has to travel a round trip Manila to Singapore and back (1485 mi apart) in a total time of 7 hours and 10 minutes. The plane from manila to Singapore is flying with a headwind of 70 mph...
-
Flexible Budget. At normal capacity, Boulder Products Corp. manufactures 10,000 trail bikes. At that level, unit variable costs for the Assembly Department are: Direct...
-
Suppose T varies directly with the 3/2 power of x. When x = 4, T = 20. Find when x = 16.
-
On January 1, 2017, McIlroy, Inc., acquired a 60 percent interest in the common stock of Stinson, Inc., for $340,200. Stinson's book value on that date consisted of common stock of $100,000 and...
-
The vapour pressure of benzene between 10C and 30C fits the expression log(p/Torr) = 7.960 1780/(T/K). Calculate (a) The enthalpy of vaporization and (b) The normal boiling point of benzene.
-
The vapour pressure of a liquid in the temperature range 200 K to 260 K was found to fit the expression ln(p/Torr) = 16.255 2501.8/(T/K). Calculate the enthalpy of vaporization of the liquid.
-
Prior to the discovery that freon-12 (CF 2 Cl 2 ) was harmful to the Earths ozone layer, it was frequently used as the dispersing agent in spray cans for hair spray, etc. Its enthalpy of vaporization...
-
A timeline of cash flows is important here. And that timeline isn't significantly different from other timelines we have looked at, except for a bunch of 0 cash flows. Summarizing, the angel investor...
-
can you do it on nike with the most current data from 2 0 2 3
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...

Study smarter with the SolutionInn App


