Question: Draw and label the given streams and derive expressions for the indicated quantities in terms of labeled variables. The solution of Part (a) is given
Draw and label the given streams and derive expressions for the indicated quantities in terms of labeled variables. The solution of Part (a) is given as an illustration.
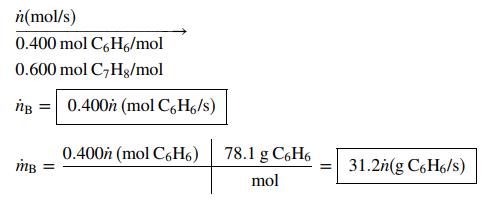
(a) A continuous stream contains 40.0 mole% benzene and the balance toluene. Write expressions for the molar and mass flow rates of benzene, ṅB(mol C6H6/s) and ṁB(kg C6H6/s), in terms of the total molar flow rate of the stream, ṅ(mol/s).
(b) The feed to a batch process contains equimolar quantities of nitrogen and methane. Write an expression for the kilograms of nitrogen in terms of the total moles n(mol) of this mixture.
(c) A stream containing ethane, propane, and butane has a mass flow rate of 100.0 g/s. Write an expression for the molar flow rate of ethane, ṅE(lb-mole C2H6/h), in terms of the mass fraction of this species, xE.
(d) A continuous stream of humid air contains water vapor and dry air, the latter containing approximately 21 mole% O2 and 79% N2. Write expressions for the molar flow rate of O2 and for the mole fractions of H2O and O2 in the gas in terms of ṅ1(lb-mole H2O/s) and ṅ2(lb-mole dry air/s).
(e) The product from a batch reactor contains NO, NO2, and N2O4. The mole fraction of NO is 0.400. Write an expression for the gram-moles of N2O4 in terms of n(mol mixture) and yNO2 (mol NO2/mol).
n(mol/s) 0.400 mol C,H6/mol 0.600 mol C,Hg/mol ng = 0.400h (mol C,H6/s) 0.400h (mol C6H6) 78.1 g C6H6 mB = 31.2h(g C6H6/s) mol
Step by Step Solution
3.40 Rating (159 Votes )
There are 3 Steps involved in it
Given that a A continues stream contains 400 mole Benzene and the balance toluene n mols 0400 mol C6 ... View full answer

Get step-by-step solutions from verified subject matter experts


