Estimate the heat of vaporization (kJ/mol) of benzene at 25C, using each of the following correlations and
Question:
Estimate the heat of vaporization (kJ/mol) of benzene at 25°C, using each of the following correlations and data:
(a) The heat of vaporization at the normal boiling point and Watson’s correlation.
(b) The Clausius–Clapeyron equation and boiling points at 50 mm Hg and 150 mm Hg.
(c) Tables B.1 and B.2.
(d) Find a tabulated value of the heat of vaporization of benzene at 25°C. (Suggestion: Do the same thing you do when you want to find almost any item of information.) Then calculate the percentage errors that result from the estimations of Parts (a), (b), and (c).
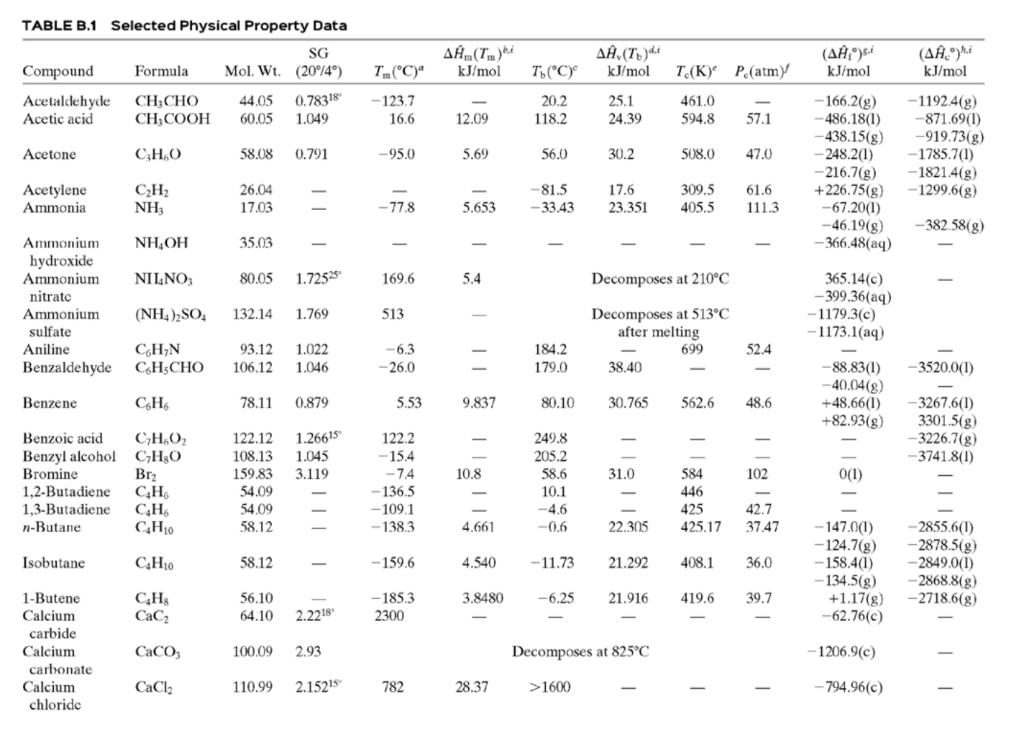
Table B.2
![TABLE B.2 Heat Capacities Form 1: C,[kJ/(mol-C)] or [kJ/(mol-K)] = a + bT + cr + dT Form 2: C,k/(molC)j or [kJ/(mol-K)j = a + bT + cT2 Example: (C,)acetone(e) = 0.07196 + (20.10 x 10-5)r - (12.78 x 10-8)7? + (34.76 x 10-12)Tr*, where T is in °C. %3D](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1590/0/8/2/6645ec6bc68ba9271590082649906.jpg)
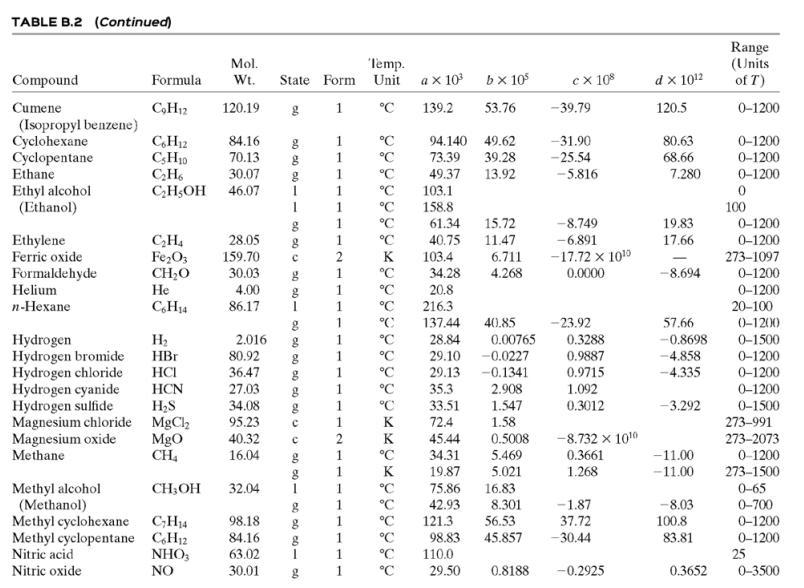
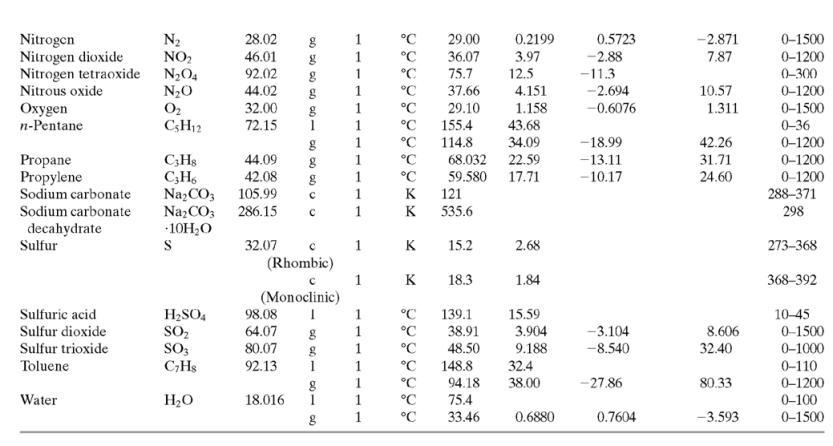
TABLE B.2 Heat Capacities Form 1: C,[kJ/(mol-C)] or [kJ/(mol-K)] = a + bT + cr + dT Form 2: C,k/(molC)j or [kJ/(mol-K)j = a + bT + cT2 Example: (C,)acetone(e) = 0.07196 + (20.10 x 10-5)r - (12.78 x 10-8)7? + (34.76 x 10-12)Tr*, where T is in °C. %3D Note: The formulas for gases are strictly applicable at pressures low enough for the ideal-gas equation of state to apply. Range (Units of T) Mol. Temp. State Form Unit a x 10 ex 10 d x 1012 Compound Formula Wt. bx 10 Acetone CH;COCH; 58.08 1 °C 123.0 18.6 -30-60 °C 71.96 34.76 20.10 6.053 0.4147 -12.78 -5.033 0.3191 0-1200 0-1200 0-1500 C;H2 26.04 29.0 °C 42.43 28.94 28.09 18.20 -1.965 1.965 -6.686 Acetylene Air 0.1965 0.4799 273-1800 Ammonia NH, 17.03 1 °C 35.15 2.954 0.4421 0-1200 Ammonium sulfate Benzene (NH4),SO4 132.15 C,H, 215.9 275-328 6-67 126.5 74.06 78.11 1 °C 23.4 32.95 -25.20 77.57 0-1200 CH10 CH10 CHs CaC2 CACO, Ca(OH)2 CaO Isobutane 58.12 89.46 49.87 30.13 27.88 -18.91 0-1200 0-1200 n-Butane 58.12 92.30 -15.47 34.98 Isobutene 56.10 82.88 25.64 -17.27 50.50 0-1200 Calcium carbide Calcium carbonate Calcium hydroxide 64.10 68.62 -8.66 x 1010 -12.87 x 1010 2 K 1.19 298-720 100.09 K 82.34 89.5 4.975 273-1033 74.10 K 276-373 -4.52 x 1010 -4.891 x 1010 -2.887 Calcium oxide 56.08 12.01 K 41.84 2.03 273-1173 Carbon с 11.18 1.095 273-1373 CO2 CO Carbon dioxide 44.01 36.11 4.233 7.464 0-1500 Carbon monoxide 28.01 1. 28.95 0.4110 0.3548 -2.220 0-1500 Carbon tetrachloride CC4 Ch 153.84 93.39 12.98 1.367 0.6117 273-343 0-1200 273-1357 Chlorine 70.91 1 °C 33.60 -1.607 6.473 Соpper Cu 63.54 1. K 22.76 212 21-
Step by Step Answer:
a b c d The heat of vaporization at normalt b p of benzene is 3072 kJm2 ieat 1009c 35...View the full answer



Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Related Video
The experiment aims to show the impact of various beverages on teeth by using eggs as a representation of enamel. Three eggs are boiled and then placed in glasses filled with fizzy drinks, vinegar, and mango juice for 24 hours. The shells of eggs are similar to enamel as they are composed of calcium carbonate, and enamel is primarily made of calcium phosphate. The eggs are then observed to demonstrate the effects of the different liquids on teeth and the importance of brushing regularly. The egg placed in fizzy drink has turned dark in color but can be cleaned by brushing with toothpaste and rinsing with water. The egg placed in vinegar has had its shell softened due to the chemical reaction of vinegar and calcium carbonate, which can\'t be reversed. This highlights the fact that acids are more damaging to teeth than other substances. The egg placed in mango juice represents the process of bacteria in the mouth converting sugars and starches into acids that form plaque, which can be prevented by brushing. The use of fluoride in toothpaste is also highlighted as it slows down the demineralization process and protects the enamel. The importance of brushing teeth twice a day is emphasized.
Students also viewed these Sciences questions
-
Estimate the heat of vaporization (kJ/mol) of benzene at a pressure of 100 mm Hg, using each of the following correlations and data: (a) The heat of vaporization at the normal boiling point given in...
-
Estimate the heat of vaporization of ethyl benzene at its normal boiling point using Troutons rule and Chens rule and compare the results with a tabulated value of this quantity. Then estimate Hv at...
-
The heat of vaporization of water at the normal boiling point, 373.2 K, is 40.66 kJ/mol. The specific heat capacity of liquid water is 4.184 JK-1g-1 and of gaseous water is 2.02 J K-1g-1. Assume that...
-
Using Table 1.6, write a structural formula for each of the following: a. An alcohol, C3H8O b. An ether, C4H10O c. An aldehyde, C3H6O d. A ketone, C3H6O e. A carboxylic acid, C3H6O2 f. An ester,...
-
Given the following reactions and their enthalpies: a. Devise a way to calculate H for the reaction H2O(g) 2H(g) + O(g) b. From this, estimate the H - O bond energy. (kJ/mol) +436 +495 H2(g)- 2H(g)...
-
Construct (using appropriate assumptions) a mixed group structure, bringing together a holding entity, a subsidiary and a sub-subsidiary.
-
Jack and Jill go for a walk along an abandoned railroad track. Jack puts one ear next to a rail, while Jill, 200 m away, taps on the rail with a stone (Figure 6.55). How much sooner does Jack hear...
-
The accountant for Save More Company made the following adjusting entries on December 31, 2011. Further information is provided as follows: (a) Annual rent is paid in advance every October 1. (b)...
-
Please fill out sheet. Answers already on the doc are given and right. Use the following information to compute Jane Smith's 2020 federal income tax payable amount or tax refund amount. Complete your...
-
The shareholders equity section of Cadmium Corporation as at December 31, 2020, follows: 8% cumulative preferred shares, 100,000 shares authorized, 80,000 shares outstanding...
-
In gas adsorption a vapor is transferred from a gas mixture to the surface of a solid. (See Section 6.7.) An approximate but useful way of analyzing adsorption is to treat it simply as condensation...
-
A gas stream containing n-hexane in nitrogen with a relative saturation of 90% is fed to a condenser at 75C and 3.0 atm absolute. The product gas emerges at 0C and 3.0 atm at a rate of 746.7 m 3 /h....
-
With few exceptions, automobile emissions standards are imposed uniformly under the Clean Air Act, which leads to an inefficient outcome. Suppose a uniform abatement standard (A ST ) has been set for...
-
A particular brand of tires claims that its deluxe tire averages at least 50,000 miles before it needs to be replaced. From past studies of this tire, the standard deviation is known to be 8,000. A...
-
Quell Company operates three segments. Income statements for the segments imply that profitability could be improved if Segment A were eliminated. Required: a. Explain the effect on profitability if...
-
Lei, an accounting major, and Jim, a marketing major, are watching a Matlock rerun on latenight TV. Of course, there is a murder and the suspect wants to hire Matlock as the defense attorney. Matlock...
-
The following events apply to 2011, the first year of operations of Shay Services: 1. Acquired \(\$ 25,000\) cash from the issue of common stock. 2. Paid \(\$ 18,000\) cash in advance for a one-year...
-
Refer to the information for Gotta Cotta in PB 10-5. Required: Compute the following for Gotta Cotta: 1. Fixed overhead spending variance 2. Fixed overhead volume variance. 3. Over or underapplied...
-
Three stars, each with the mass and radius of our sun, form an equilateral triangle 5.0 10 9 m on a side. If all three are simultaneously released from rest, what are their speeds as they crash...
-
An environmentalist wants to determine if the median amount of potassium (mg/L) in rainwater in Lincoln County, Nebraska, is different from that in the rainwater in Clarendon County, South Carolina....
-
Most common amines (RNH 2 ) exhibit pK a values between 35 and 45. R represents the rest of the compound (generally carbon and hydrogen atoms). However, when R is a cyano group, the pK a is found to...
-
Provide a systematic name for each of the following compounds: a. b. c. d.
-
Compound X has molecular formula C 7 H 14 . Hydrogenation of compound X produces 2, 4-dimethylpentane. Hydroboration-oxidation of compound X produces a racemic mixture of 2, 4-dimethylpentan-1-ol...
-
Management of Mittel Rhein AG of Kln, Germany, would like to reduce the amount of time between when a customer places an order and when the order is shipped. For the first quarter of operations...
-
Of the following, which form of collateral would score the highest when measured against the MAST framework? Review Later Inventory Bonds Machinery Intellectual property
-
1 . Hadley Corporation, which has only one product, has provided the following data concerning its most recent month of operations: Selling price$ 2 1 1 Units in beginning inventory 2 0 0 Units...

Study smarter with the SolutionInn App


