Explain the direction of the chemical shifts for Fe(0), Fe(II), and Fe(III) in Figure 22.20. Fe 2p
Question:
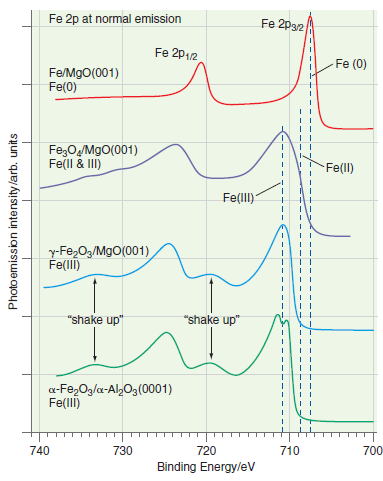
Transcribed Image Text:
Fe 2p at normal emission Fe 2p32 Fe 2p12 Fe (0) Fe/MgO(001) Fe(0) Fe;OM9O(001) Fe(ll & II) Fe(ll) Fe(lI) y-Fe,O3/MgO(001) Fe(llI) "shake up" "shake up" a-Fe,Og/a-Al2Og (0001) Fe(llI) 740 730 720 710 700 Binding Energy/ev Photo emission intensity /arb. units
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
As electrons are removed from Fe to form Fe 2 or Fe 3 ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict the chemical shifts of the protons in the following compounds. (a) (b) (c) (d) (e) (f) HC(CH3)3 CH CH CH3 , - CH,CCCH OH CH,CH,-C-OH CH3 CHC-CH Br
-
The proton NMR spectrum of 2-pyridone gives the chemical shifts shown. (a) Is 2-pyridone aromatic? (b) Use resonance forms to explain your answer to (a). Also explain why the protons at (7.31and...
-
The chemical shifts of the 1H nuclei in 2,2-dimethylpropane and TMS are 0.95 and 0.0, respectively. From these data, what can you deduce about the relative electronegativities of carbon and silicon?
-
A non reactive/conservative contaminant is dumped on the ground level and it leaches to the groundwater vertically and takes half day for reaching the groundwater by travelling through unsaturated...
-
The point is on the terminal side of an angle in standard position. Find the exact values of the six trigonometric functions of the angle. 1. (12, 16) 2. (7, -24) 3. (-0.5, 4.5)
-
Let f (x) = ax + b and g(x) = bx + a, where a and b are integers. If f (1) = 8 and f (g(20)) g(f (20)) = 14, find the product of a and b.
-
Measuring the moons orbit. Refer to the American Journal of Physics (Apr. 2014) study of the moons orbit, Exercise 11.23 (p. 626). Recall that the angular size (y) of the moon was modeled as a...
-
Lindex Company uses a process costing system. The following data are available for one department for October: The department started 390,000 units into production during the month and transferred...
-
Q4. After saving lots of money for her dream venture, Divya finally opened her art venture. However, since she never paid attention in her accounting classes, she is clueless on how to assess the...
-
You, CPA, are working as the controller for a video game development company called All Starr Games Inc. (All Starr). The company develops sports-related games, and its recent virtual rugby game was...
-
The principal line in the emission spectrum of sodium is yellow. On close examination, the line is seen to be a doublet with wavelengths of 589.0 and 589.6 nm. Explain the source of this doublet.
-
Why is XPS a surface-sensitive technique?
-
What factors determine whether a person is an employee or an independent contractor?
-
Before beginning a study investigating the ability of the drug heparin to prevent bronchoconstriction, baseline values of pulmonary function were measured for a sample of 12 individuals with a...
-
which of the following (list all that apply) are advantages of a balanced binary search tree over an unbalanced one: 1. it requires less memory 2. it's faster to move from node to node 3. it's faster...
-
6) Do you find conditional probability problems challenging? Have you tried watching the videos on canvas and has it helped?
-
1. Determine the cost of heating 3 gallons of water (water weighs 8.33L per gallon ) at a room temperature of 22 degrees Celsius to the boiling point of 100 degrees Celsius at the energy rating of...
-
Writer One Inc. manufactures ball point pens that sell at wholesale for $0.80 per unit. Budgeted production in both 2018 and 2019 was 16,000 units. There was no beginning inventory in 2018. The...
-
In Exercises 3952, a. Find an equation for f -1 (x). b. Graph f and f -1 in the same rectangular coordinate system. c. Use interval notation to give the domain and the range of f and f -1 . f(x) = (x...
-
At 31 December 20X9, the end of the annual reporting period, the accounts of Huron Company showed the following: a. Sales revenue for 20X9, $ 2,950,000, of which one- quarter was on credit. b....
-
The heat capacity of chloroform (trichloromethane, CHCl 3 ) in the range 240 K to 330 K is given by C p,m /(J K 1 mol 1 ) = 91.47 + 7.5 10 2 (T/K). In a particular experiment, 1.00 mol CHCl 3 is...
-
A sample consisting of 1.0mol CaCO 3 (s) was heated to 800C, when it decomposed. The heating was carried out in a container fitted with a piston that was initially resting on the solid. Calculate the...
-
A chemical reaction takes place in a container of cross-sectional area 100 cm 2 . As a result of the reaction, a piston is pushed out through 10 cm against an external pressure of 1.0 atm. Calculate...
-
ABC company makes turbo-encabulators, customized to satisfy each customers order. They split overhead into five pools, each with its own activity driver (direct labor for manufacturing, direct labor...
-
Variable manufacturing overhead becomes part of a unit's cost when variable costing is used.Group of answer choicesTrueFalse
-
Santa Fe Corporation has computed the following unit costs for the year just ended:Direct Material used $23Direct Labor $18Fixed selling and administrative cost $18Variable manufacturing overhead...

Study smarter with the SolutionInn App


