For a gas that obeys the equation of state V m = Rt/P + B(T) derive the
Question:
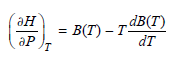
Transcribed Image Text:
dB(T) = B(T) – Tª ән т dт ӘР
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (15 reviews)
Equation 344 ...View the full answer

Answered By

Mehwish Aziz
What I have learnt in my 8 years experience of tutoring is that you really need to have a friendly relationship with your students so they can come to you with their queries without any hesitation. I am quite hardworking and I have strong work ethics. Since I had never been one of those who always top in the class and always get A* no matter what, I can understand the fear of failure and can relate with my students at so many levels. I had always been one of those who had to work really hard to get decent grades. I am forever grateful to some of the amazing teachers that I have had who made learning one, and owing to whom I was able to get some extraordinary grades and get into one of the most prestigious universities of the country. Inspired by those same teachers, I am to be like one of them - who never gives up on her students and always believe in them!
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Find an expression for the fugacity coefficient of a gas that obeys the equation of state pVm = RT(1 +B/Vm + C/V-1). Use the resulting expression to estimate the fugacity of argon at 1.00 am3 and 100...
-
Derive relations for (a) u, (b) h, and (c) s of a gas that obeys the equation of state (P + a/v 2)v = RT for an isothermal process.
-
Derive relations for (a) u. (b) h. (c) s of a gas that obeys the equation of state (P + a/v2)v = RT for an isothermal process.
-
All numbers are in $ '000. Consider an income property. Next three years its NOIs will be $25,000, $28,000 and $30,000. Then NOI will be growing at a constant rate of 3% per year. If you buy the...
-
You're leading a project to migrate customer data (biographical, billing, and orders) from the legacy system to an off-the-shelf product sold by MicroTech. The project was going well, but a key...
-
Factor the given expressions completely. a 2 x 2 + a 2
-
What is the signifi cance of the S-shape of the sales response function in Figure 221?
-
a. Identify the main determinants for valuation of feature films, television programs, and general release feature productions by Columbia Pictures. b. Are the bases of valuation reasonable? Explain....
-
XYZ international Pvt Ltd . A manufacturing company of drugs, is considering switching from their traditional method of cost to activity - based costing. The two products Z - Serum and W - serum can...
-
Consider a CMOS process for which Lmin = 0.25 m, tox = 6 nm, n 460 cm2/V . s, and V; %3| 0.5 V. (a) Find Cox and k', (b) For an NMOS transistor with W/L = 15 um/0.25 um, calculate the values of Voy ,...
-
What is sustainable water use?
-
What can you say about H vaporization of a liquid as the temperature approaches the critical temperature?
-
Find P 99 , the 99th percentile. This is the bone density score separating the bottom 99% from the top 1%. Assume that a randomly selected subject is given a bone density test. Bone density test...
-
How have your organizations performed relative to improving healthcare quality and meeting the required standards (Medicare metrics) for value-based purchasing initiatives?
-
/ Precalculus Algebra Problem. 1: Consider the function f(x)=-5x5 + +-4. How many terms in f(x) are not monomials? Problem. 2: Consider the function f(x)=-3x-4x - 3x + 12. How many terms in f(x) are...
-
D 0
-
What NaCl concentration results when 279 mL of a 0.680 M NaCl solution is mixed with 462 mL of a 0.450 M NaCl solution? concentration: M
-
Use JavaFX's shape's classes from javafx.scene.shape package to complete the following questions (Hint: CANNOT use any Gaphics or Graphics2D classes from java.awt packages): DO not write the whole...
-
In Exercises 45 through 48, the derivative of a function f(x) is given. In each case, find the critical numbers of f(x) and classify each as corresponding to a relative maximum, a relative minimum,...
-
The roof of a refrigerated truck compartment is of composite construction, consisting of a layer of foamed urethane insulation (t2 = 50 mm, ki = 0.026 W/m K sandwiched between aluminum alloy panels...
-
Calculate the molar volume of chlorine gas at 350 K and 2.30 atm using (a) The perfect gas law and (b) The van der Waals equation. Use the answer to (a) to calculate a first approximation to the...
-
Calculate the volume occupied by 1.00 mol N2 using the van der Waals equation in the form of a virial expansion at (a) Its critical temperature, (b) Its Boyle temperature, and (c) Its inversion...
-
The mass density of water vapour at 327.6 atm and 776.4 K is 133.2 kg m-3. Given that for water Tc = 647.4 K, Pc = 218.3 atm, a = 5.464 dm6 atm mol-2, b= 0.03049 dm3 mol-1, and M= 18.02 g mol-1,...
-
A stock is expected to pay a dividend of $1.50 at the end of the year (i.e., D 1 = $1.50), and it should continue to grow at a constant rate of 10% a year. If its required return is 14%, what is the...
-
The Hobby Shop has a checking account with a ledger balance of $1,700. The firm has $2,400 in uncollected deposits and $4,200 in outstanding checks. What is the amount of the disbursement float on...
-
An investment will pay you $34,000 in 11 years. If the appropriate discount rate is 6.1 percent compounded daily, what is the present value? (Use 365 days a year. Do not round intermediate...

Study smarter with the SolutionInn App


