From the enthalpy of combustion data in Table 2C.1 for the alkanes methane through octane, test the
Question:
From the enthalpy of combustion data in Table 2C.1 for the alkanes methane through octane, test the extent to which the relation ΔcH⦵ = k{(M/(g mol−1)}n holds and find the numerical values for k and n. Predict ΔcH⦵ for decane and compare to the known value.
Data in table 2C.1
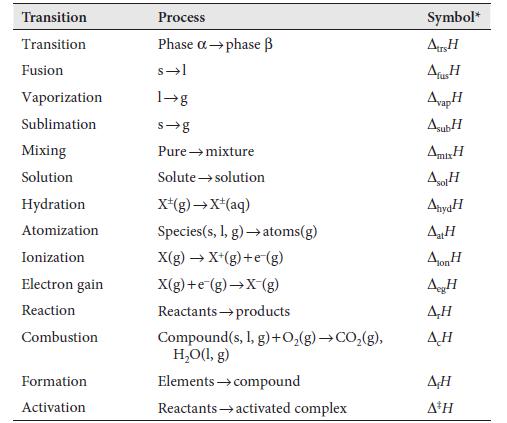
Transcribed Image Text:
Transition Transition Fusion Vaporization Sublimation Mixing Solution Hydration Atomization Ionization Electron gain Reaction Combustion Formation Activation Process Phase a →phase B s-l 1→g s-g Pure → mixture Solute → solution X*(g) →X+(aq) Species (s, 1, g) → atoms(g) X(g) → X+(g) +e (g) X(g)+e (g) →X-(g) Reactants products Compound(s, I, g) + O₂(g) →CO₂(g), H₂O(l,g) Elements → compound Reactants activated complex Symbol* Atrs H ΔΗ AvapH AsubH Amir H sol AnydH AtH Aon H AH A,H ΔΗ AH A³H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
The relation cH kMg mol1n is a power law relation that suggests the enthalpy of combustion cH is pro...View the full answer

Answered By

Abdul Wahab Qaiser
Before working at Mariakani, I volunteered at a local community center, where I tutored students from diverse backgrounds. I helped them improve their academic performance and develop self-esteem and confidence. I used creative teaching methods, such as role-playing and group discussions, to make the learning experience more engaging and enjoyable.
In addition, I have conducted workshops and training sessions for educators and mental health professionals on various topics related to counseling and psychology. I have presented research papers at conferences and published articles in academic journals.
Overall, I am passionate about sharing my knowledge and helping others achieve their goals. I believe that tutoring is an excellent way to make a positive impact on people's lives, and I am committed to providing high-quality, personalized instruction to my students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula
Question Posted:
Students also viewed these Sciences questions
-
The enthalpy of combustion of benzoic acid (C6H5COOH) is commonly used as the standard for calibrating constant-volume bomb calorimeters; its value has been accurately determined to be -3226.7...
-
The enthalpy of combustion of hard coal averages 351 kJ/g, that of gasoline, 1.33 10 5 kJ/gal. How many kilograms of hard coal provide the same amount of heat as is available from 120 gallon of...
-
The enthalpy of combustion of glucose (C 6 H 12 O 6 ) is roughly 15.6 MJ/kg. Compute the fraction of incident solar energy from 8 photons with wavelength 680 nm stored through the reaction (26.2) in...
-
You work in the finance department of a telecommunications firm with a large direct sales force selling high- speed fiber optics access lines to companies wanting telephone and Internet access. Your...
-
Saxon Woods, Inc. has a fiscal year- end of December 31, 2015. The company reported $ 124,500 in short- term notes payable due on April 1, 2016, on its year- end balance sheet. Saxon Woods extended...
-
The enrollments of the 13 public universities in the state of Ohio for the 201718 academic year are listed below. University Main and Regional Campuses Enrollment University of Akron . . . . . . . ....
-
What does a resource dependency theorist say about organization survival? LO.1
-
Walker Company has 15,000 shares of common stock outstanding during all of 2007. It also has two convertible securities outstanding at the end of 2007. These are: 1. Convertible preferred stock:...
-
4/ You are proposing a new project that involves buying a new grinding machine. You are confident in your estimates of costs and returns but are concerned that the salvage value of the machine at the...
-
1. Based on what you read in this chapter, what would you have suggested Lisa and her team do first with respect to training, particularly in terms of the companys strategy? Why? 2. Have Lisa and the...
-
Why is the heat capacity at constant pressure of a substance normally greater than its heat capacity at constant volume?
-
Estimate the internal pressure, T , of water vapour at 1.00 bar and 400K, treating it as a van der Waals gas.
-
Pizza Places records include the following accounts with regards to purchases of plastic glasses as at 31 December of the current year: By 31 December the company sold 10,100 units for 154,876....
-
The curved rod has a diameter \(d\). Determine the vertical displacement of end \(B\) of the rod. The rod is made of material having a modulus of elasticity of \(E\). Consider only bending strain...
-
If the inertial measurement system were written in C++ according to the design fragment described in Chapter 5, describe the testing strategy you would use. If possible, try to design some test cases.
-
Determine the displacement at point \(C\) of the W14 \(\times 26\) beam made from A992 steel. 8 kip A -5 ft 5 ft. B C -5 ft 5 ft- 8 kip D
-
The beam is subjected to the loading shown. Determine the slope at \(B\) and displacement at \(C\). \(E I\) is constant. Ta Mo C b B
-
A mass, connected to a damper as shown in Fig. 14.30, is subjected to a force \(F(t)\). Find the frequency-response function \(H(\omega)\) for the velocity of the mass. m F(t) y(1) FIGURE 14.30...
-
Thorium-232 undergoes alpha decay. Complete the reaction equation for this decay and identify the daughter nucleus. 90 Th 232 ? +
-
APC16550D UART has a clock running at18.432 MHz and its baud rate is set to 2000.Determine the HEX contents of its DLM and DLL registers. Please can you explain step by step and in detail how you get...
-
The electronic absorption bands of many molecules in solution have half-widths at half-height of about 5000 cm 1 . Estimate the integrated absorption coefficients of bands for which (a) max 1 104...
-
A swimmer enters a gloomier world (in one sense) on diving to greater depths. Given that the mean molar absorption coefficient of sea water in the visible region is 6.2 10 3 dm 3 mol 1 cm 1 ,...
-
The compound CH 3 CH=CHCHO has a strong absorption in the ultraviolet at 46 950 cm 1 and a weak absorption at 30 000 cm 1 . Justify these features in terms of the structure of the molecule.
-
solve this plz Alba Company is considering the introduction of a new product. To determine the selle price of the product you have The direct material permit The direct labor per unit The variable...
-
Calculate the current ratio collection period for accounts receivable, inventory turnover, gross margin percentage, and return on equity for 2014 and 2015 for the Jordan Corporation. Do not average....
-
A company received $11,000 cash in exchange for 200 shares of the companys common stock. What would the effect of this transaction on the current years accounting equation? Select one: A. No effect...

Study smarter with the SolutionInn App


