From the following data, derive the absolute entropy of crystalline glycine at T = 300.K. You can
Question:
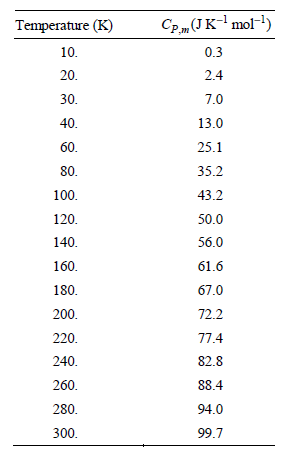
Transcribed Image Text:
CP„(JK-mol-) Temperature (K) 10. 0.3 20. 2.4 30. 7.0 13.0 40. 60. 25.1 35.2 80. 100. 43.2 50.0 120. 140. 56.0 160. 61.6 180. 67.0 72.2 200. 220. 77.4 82.8 240. 260. 88.4 280. 94.0 99.7 300.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
The line in the graph above of C Pm vertical axis against T is the best fit to the ...View the full answer

Answered By

Pranav Makode
I am a bachelor students studying at professor ram meghe institute of technology and research. I have a great experience of being an expert. I have worked as an expert at helloexperts and solvelancer as a part time job. I have also worked as a doubt solver at ICAD SCHOOL OF LEARNING, which is in Amravati city. I have also worked as an Freelancer.
I have great experience of helping students, as described above. I can help any students in a most simple and understandable way. I will not give you have any chance for complaint. You will be greatfull to accept me as an expert.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
From the following data, determine f Ho for diborane, B2H6 (g), at 298 K: (I) B2H6 (g) + 3 O2 (g) B2O3(s) + 3 H20 (g) t Ho=-1941 kJ mol-1 (2) 2 B(s) + t Oh) B2O3(S) t, Ho = -2368 kJ mol-1 (3) H2 (g)...
-
From the following data for liquid nitric acid, determine its heat of vaporization and normal boiling point. Temperature C) Vapor Pressure (mm Hg) 10. 20. 30. 40. 50. 80. 14.4 26.6 47.9 81.3 133 208...
-
From the following data for three prospective fuels, calculate which could provide the most energy per unit volume: Density at 20 C Molar Enthalpy of Combustion Fuel (g/cm (kJ/mol) Nitroethane, C2H...
-
Understand the content theories of motivation.
-
Why are slow-moving items dangerous to the small business? What can be done to liquidate them from inventory?
-
A communications satellite remains stationary at an altitude of 22,500 mi over a point on Earths equator. It therefore rotates once each day about Earths center. Its velocity is constant, but the...
-
A right to know? Some people think that the law should require that all political poll results be made public. Otherwise, the possessors of poll results can use the information to their own...
-
The following selected accounts and their current balances appear in the ledger of Kanpur Co. for the fiscal year ended June 30, 2016: Instructions 1. Prepare a single-step income statement in the...
-
Suppose the following items were taken from the 2017 financial statements of Vaughn Manufacturing . (All dollars are in millions.) Common stock $3,400 Accumulated depreciationequipment $4,050 Prepaid...
-
Regal Entertainment Group is the largest movie company in the world, taking in over 20 percent of the box office receipts in the United States. Listed here are transactions that typically occur each...
-
How many finished units per day can the current assembly process produce? Where are the bottlenecks?
-
If the process needed to make 18 units per day, what should you do?
-
Suppose the fraction of individuals with some superior gene increases by 10% each generation. a. Write the discrete-time dynamical system for the fraction of organisms with the gene (denote the...
-
Create your own privacy philosophy. This should cover the policies you will use for email, texting, social media, and internet usage. Consider what information is being collected anout you in each...
-
1. What future markets might be attractive to Carrefour and which mode of operation would be preferable? How important is the theoretical concept of psychological distance? 2. Corporate...
-
Briefly restate your problem space and methodology. Considering your problem space and methodology, what factors are you considering in deciding whether to use a theoretical foundation or a...
-
In light of your personal experience, what strategies or approaches do you believe could be effective in creating a workplace environment where employees from diverse cultural backgrounds feel both...
-
1. Entrepreneurs hold many common traits, identify five common traits of an entrepreneur that resonate with you and discuss each one of the five traits and why they matter to you. 2. Why is...
-
Solve each rational inequality in Exercises 4360 and graph the solution set on a real number line. Express each solution set in interval notation. -x + 2 x - 4 0
-
Suppose that a business sells 6-month subscriptions to its monthly magazine. On January 1, the company receives a total of $600 for 10 subscriptions. To record this transaction, the company debits...
-
Solve for the hydrogen-ion concentration in solutions of acetic acid with stoichiometric molarities equal to 0.00100 mol l 1 . Use the method of successive approximations.
-
Verify the prediction of the ideal gas equation of state given in the previous example.
-
Substitute the value of the molar volume obtained in the previous example and the given temperature into the Dieterici equation of state to calculate the pressure. Compare the calculated pressure...
-
In 2019, Sunland Company had a break-even point of $388,000 based on a selling price of $5 per unit and fixed costs of $155,200. In 2020, the selling price and the variable costs per unit did not...
-
11. String Conversion Given a binary string consisting of characters '0's and '1', the following operation can be performed it: Choose two adjacent characters, and replace both the characters with...
-
Consider the table shown below to answer the question posed in part a. Parts b and c are independent of the given table. Callaway Golf (ELY) Alaska Air Group (ALK) Yum! Brands (YUM) Caterpillar...

Study smarter with the SolutionInn App


