Identify the molecular orbitals for F 2 in the images shown here in terms of the two
Question:
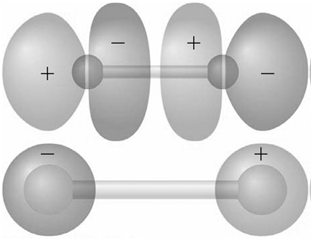
Transcribed Image Text:
+ +
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 73% (15 reviews)
The top image has symmetry the AOs are out of ...View the full answer

Answered By

Elias Gichuru
am devoted to my work and dedicated in helping my clients accomplish their goals and objectives,providing the best for all tasks assigned to me as a freelancer,providing high quality work that yields high scores.promise to serve them earnestly and help them achieve their goals.i have the needed expertise,knowledge and experience to handle their tasks.
4.80+
325+ Reviews
859+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
(a) Draw the molecular orbitals for the cyclopropenyl case. (Since there are three p orbitals, there must be three MOs: one all-bonding MO and one degenerate pair of MOs.) (b) Draw an energy diagram...
-
Evaluate the Klopp case in terms of those two criteria.
-
Images of molecular orbitals for LiH calculated using the minimal basis set are shown here. In these images, the smaller atom is H. The H1s AO has a lower energy than the Li2s AO. The energy of the...
-
One of Red Clay's employee handbook sections covers employee monitoring. An employee filed a complaint with the HR department stating he opposes this monitoring and chooses to opt out. What legal...
-
Different types of granular substances naturally settle at different angles when stored in cone-shaped piles. This angle θ is called the angle of repose (see figure). When rock salt is...
-
Circle all pronouns and rewrite to correct any pronoun errors. Caroline passed the phone to Julia, but she couldnt bring herself to speak.
-
New method of estimating rainfall. Accurate measurements of rainfall are critical for many hydrological and meteorological projects. Two standard methods of monitoring rainfall use rain gauges and...
-
A completed worksheet for The King Group is shown below. INSTRUCTIONS 1. Record balances as of December 31, 2019, in the ledger accounts. 2. Journalize (use 3 as the page number) and post the...
-
Please answer A and B A project in South Korea requires an initial investment of 3 billion South Korean won. The project is expected to generate net cash flows to the subsidiary of 5 billion and 7...
-
A current of 0.20 A flows in a wire of length 2.50 m placed at right angles to a magnetic field of flux density 0.060 T. Calculate the force on the wire.
-
Explain why the nodal structures of the 1 g MOs in H 2 and F 2 differ.
-
In discussing Figure 23.2, the following statement is made: Interchanging red and blue does not generate a different MO. Justify this statement. Figure 23.2 H1s His H2 Energy
-
Draw the resonance contributors of the cyclooctatrienyl dianion. a. Which of the resonance contributors is the least stable? b. Which of the resonance contributors makes the smallest contribution to...
-
Admin Support Cereal Bars Square Foot 1,250 1,500 7,500 7,000 # of employees 14 11 42 59 # of machine batches 0 0 14 27 # of computers 17 21 35 30 Costs 32,000.32 21,740.21 The Support department...
-
Compare and contrast the differences between innovation and creativity. Does one lead to the other? If so, please explain. Why is innovation important? Who within the organization is responsible for...
-
Using the tables from Check your Consumer Surplus and Producer Surplus activities, find the equilibrium price and quantity in the market for cheese-stuffed jalapeno peppers. What is the total surplus...
-
We decided to use Gehan's two-stage design for this purpose. In the first stage, we will discard the new treatment if no patient out of n0 patients. Suppose the probability we can tolerate to discard...
-
Claude Haridge was involved in a demonstration. He threw a paint balloon at a bus and some of the paint flecks hits a nearby officer, so Haridge was transported to police cells. At the cells Special...
-
In Exercises 5564, use the vertical line test to identify graphs in which y is a function of x. X
-
The Dow Jones Industrial Average reached a high of $ 7801.63 on December 29, 1997. Recall from Example 18.4 that it reached a high of $ 1003 on November 14, 1972. The Consumer Price Index for...
-
Discuss the origins of diagonal and cross peaks in the COSY spectrum of an AX system.
-
Calculate the frequency separation of the nuclear spin levels of a 13 C nucleus in a magnetic field of 14.4 T given that the magnetogyric ratio is 6.73 10 7 T 1 s 1 .
-
33 S has a nuclear spin of 3/2 and a nuclear g-factor of 0.4289. Calculate the energies of the nuclear spin states in a magnetic field of 7.500 T.
-
In 2019, Sunland Company had a break-even point of $388,000 based on a selling price of $5 per unit and fixed costs of $155,200. In 2020, the selling price and the variable costs per unit did not...
-
11. String Conversion Given a binary string consisting of characters '0's and '1', the following operation can be performed it: Choose two adjacent characters, and replace both the characters with...
-
Consider the table shown below to answer the question posed in part a. Parts b and c are independent of the given table. Callaway Golf (ELY) Alaska Air Group (ALK) Yum! Brands (YUM) Caterpillar...

Study smarter with the SolutionInn App


