In the manufacture of pharmaceuticals, most active pharmaceutical ingredients (APIs) are made in solution and then recovered
Question:
In the manufacture of pharmaceuticals, most active pharmaceutical ingredients (APIs) are made in solution and then recovered by separation. Acetaminophen, a pain-killing drug commercially marketed as Tylenol®, is synthesized in an aqueous solution and subsequently crystallized. The slurry of crystals is sent to a centrifuge from which two effluent streams emerge: (1) a wet cake containing 90.0 wt% solid acetaminophen (MW = 151 g/mol) and 10.0 wt% water (plus some acetaminophen and other dissolved substances, which we will neglect), and (2) a highly dilute aqueous solution of acetaminophen that is discharged from the process.
The wet cake is fed to a dryer where the water is completely evaporated, leaving the residual acetaminophen solids bone dry. If the evaporated water were condensed, its volumetric flow rate would be 50.0 L/h. Following is a flowchart of the process, which runs 24 h/day, 320 days/yr. A denotes acetaminophen.
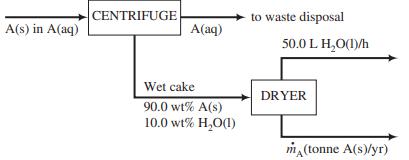
(a) Calculate the yearly production rate of solid acetaminophen (tonne/yr), using as few dimensional equations as possible.
(b) A proposal has been made to subject the liquid solution leaving the centrifuge to further processing to recover more of the dissolved acetaminophen instead of disposing of the solution. On what would the decision depend?
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





