Is the expression only valid for an ideal gas if V is constant? T, AUr = [
Question:
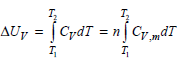
only valid for an ideal gas if V is constant?
Transcribed Image Text:
T, AUr = [ CydT = n[ Cy,maT
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
No It is always valid for an ...View the full answer

Answered By

Muhammad Mahtab
everyone looks that their work be perfect. I have more than a five year experience as a lecture in reputable institution, national and international. I provide perfect solution in marketing, case study, finance problems, blog writing, article writing, business plans, strategic management, human resource, operation management, power point presentation and lot of clients need. Here is right mentor who help clients in their multi-disciplinary needs.
5.00+
3+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Why is the equation
-
Is the equation valid for an ideal gas? Tf PV; -V;) T; Cy dT Lav = C, n2v, -v) %3D AS =
-
Show that for an ideal gas cp = cv + R u.
-
Each of the following passages may be plausibly criticized by some who conclude that it contains a fallacy, but each may be defended by some who deny that the argument is fallacious. Discuss the...
-
When reviewing a balance sheet of a corporation, what are things or items that indicate a company is in good financial health? What items indicate poor financial health? How can these concepts be...
-
Find f when p = 2 and c = 4 if f varies jointly as p and the cube of c, and f = 8 when p = 4 and c = 0.1.
-
Of the four growth strategies described in the chapter, which is the riskiest? Which is the easiest to implement? Why?
-
General Disclosures; Inventories; Property, Plant, and Equipment Koch Corporation is in the process of preparing its annual financial statements for the fiscal year ended April 30, 2011. Because all...
-
Sherrie Company purchased equipment and the following costs were associated with the purchase: Price of the equipment $87,000 Sales taxes $2,700 Shipping Insurance $540 Annual Maintenance Costs...
-
Peterman Inc. provides an automobile to Ms. Winters for her to use in carrying out her employment duties. Ms. Winters is given full possession of the car and is allowed to use it for personal needs....
-
For each pair of compounds below, identify the one that would be expected to have more ionic character. Explain your choice. a) NaBr or HBr b) BrCl or FCl
-
Draw a Lewis dot structure for each of the following compounds: a. CH 3 CH 2 OH b. CH 3 CN
-
MWC Corp. is currently in the sixth year of its existence (2014). In 2009-2013, it reported the following income and (losses) (before net operating loss carryovers or carrybacks)....
-
Nelsie Corporation has an outstanding 60-day 6% note receivable amounting to P 15,000 dated December of the ne year. The company is using the calendar year in preparing its financial statements. What...
-
Which resource is the bottleneck? What is the overall capacity of the orthopedist's office in patients/hour?
-
What are the comprehensive strategic implentation issues of Kmart with reference
-
1. Create both the written plan and the educational material to help African American women age 65+ control high blood pressure, take the special circumstances into consideration for the plan. 2. For...
-
Write down D & S equations for wireless phones; include two exogenous variables in each equation.
-
In Exercises 5166, find a. (f g)(x) b. (g f)(x) c. (f g)(2) f(x) = 4x - 3, g(x) = 5x 2 - 2
-
Suppose that the laptop of Prob. 2.16 is placed in an insulating briefcase with a fully charged battery, but it does not go into sleep mode, and the battery discharges as if the laptop were in use....
-
The effective rate constant for a gaseous reaction that has a Lindemann-Hinshelwood mechanism is 1.7 X 10-3 s-I at 1.09 kPa and 2.2 X 10-4 S-1 at 25 Pa. Calculate the rate constant for the activation...
-
The data below apply to the formation of urea from ammonium cyanate, NH4CNO --7 NH2CONH2. Initially 22.9 g of ammonium cyanate was dissolved in enough water to prepare 1.00 dm3 of solution. Determine...
-
The thermal decomposition of an organic nitrile produced the following data: Determine the order of the reaction and the rate constant. t/(10's) 0 2.00 4.00 6.00 8.00 10.00 12.00 [nitrile]/(mol dm)...
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...

Study smarter with the SolutionInn App


