Nitrogen is a vital element for all living systems, but except for a few types of bacteria,
Question:
A possible pathway for ammonia synthesis by a living system is:
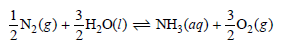
where (aq) means the ammonia is dissolved in water and ˆ†Gof(NH3, aq) = -80.3 kJ mol-1
a. Calculate ΔG° for the biological synthesis of ammonia at 298 K.
b. Calculate the equilibrium constant for the biological synthesis of ammonia at 298 K.
c. Based on your answer to part (b), is the pathway a spontaneous reaction?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





