Oxygen consumed by a living organism in aerobic reactions is used in adding mass to the organism
Question:
Oxygen consumed by a living organism in aerobic reactions is used in adding mass to the organism and/or the production of chemicals and carbon dioxide. Since we may not know the molecular compositions of all species in such a reaction, it is common to define the ratio of moles of CO2 produced per mole of O2 consumed as the respiratory quotient, RQ, where
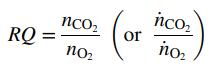
Since it generally is impossible to predict values of RQ, they must be determined from operating data. Mammalian cells are used in a bioreactor to convert glucose to glutamic acid by the reaction
![]()
The feed to the bioreactor comprises 1.00x102 mol C6H12O6 /day, 1.20x102 mol NH3 /day, and 1.10x102 mol O2/day. Data on the system show that RQ = 0.45 mol CO2 produced/mol O2 consumed.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





