Some polymers can form liquid crystal mesophases with unusual physical properties. For example, liquid crystalline Kevlar (3)
Question:
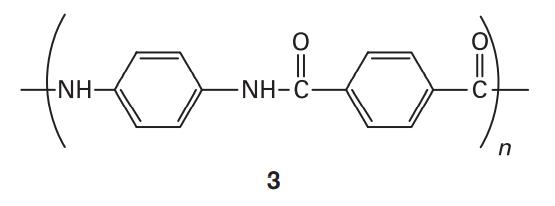
Some polymers can form liquid crystal mesophases with unusual physical properties. For example, liquid crystalline Kevlar (3) is strong enough to be the material of choice for bulletproof vests and is stable at temperatures up to 600 K. What molecular interactions contribute to the formation, thermal stability, and mechanical strength of liquid crystal mesophases in Kevlar?
Transcribed Image Text:
O formam -NH- -NH-C 3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
Solution Liquid crystal Kevlar is a network polymer with molecula...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Some polymers can form liquid crystal mesophases with unusual physical properties. For example, liquid crystalline Kevlar (1) is strong enough to be the material of choice for bulletproof vests and...
-
Some polymers have unusual properties. For example, Kevlar (3) is strong enough to be the material of choice for bulletproof vests and is stable at temperatures up to 600K. What molecular...
-
The American Aluminum Company is considering making a major investment of $150 million ($5 million for land, $45 million for buildings, and $100 million for manufacturing equipment and facilities) to...
-
Joe rents his condo for $1,500 per month. Total rental and personal use days for the current year was 210 days and 20 days, respectively. What are the tax consequences for Joe?
-
What is the OTC market for trading derivatives? How do OTC markets differ from exchanges?
-
Do you believe that creativity is an in-born trait that only a few people have? If so, why? If not, how would you encourage creativity in your followers?
-
For typical cameras, the f-number can be selected from a set of fixed values such as 2, 2.8, 4, 5.6, 8, 11, and 16. Explain why this combination of f-numbers is used. The diameter of the aperture is...
-
Atlantic Academy is a private school that offers education to children from Kindergarten to Grade 7. The school operates as a not-for-profit entity and oversight of the school is performed by the...
-
Which type of analysis shows the income statement amounts as a percentage of net sales? A. Horizontal B. Vertical C. Trend
-
Ewing Limited is trying to determine the value of its ending inventory as of February 28, 2010, the companys year-end. The following transactions occurred, and the accountant asked your help in...
-
Hexane and perfluorohexane show partial miscibility below 22.70C. The critical concentration at the upper critical temperature is x = 0.355, where x is the mole fraction of C 6 F 14 . At 22.0C the...
-
Refer to the information in Exercise 6.15(b) and sketch the cooling curves for liquid mixtures in which x(B 2 H 6 ) is (a) 0.10, (b) 0.30, (c) 0.50, (d) 0.80, and (e) 0.95. Data in Exercise 6.15(b)...
-
Visit the PCOAB website (i.e., www.pcaobus.org), search for the tip and referral center and review the guidelines. Can you report a violation to the PCAOB anonymously? Assuming that the employee knew...
-
Jennifer purchased stock at $50 per share with a 75% initial margin requirement and a maintenance margin of 35%. How much equity per share must Jennifer contribute when the stock falls to $15 per...
-
Thinking about your present job and your "inventory"of leadership traits and characteristics, where are your strengths and weaknesses as a leader?Is being a leader desirable? If yes, what motivates...
-
You are facing a complex decision with several courses of possible action and probabilities associated with them. The current decision tree, based on the best possible estimates of probabilities and...
-
1. In what ways has Marriot proven an industry leader in the context of entrepreneurship in the hospitality industry. 2. What are the author's metrics of measuring entrepreneurial activity, and do...
-
Suppose you want to model the relationship between the interest rate, the economic growth rate and the inflation rate. what would be first model to fit explain.
-
Suppose you find the linear approximation to a differentiable function at a local maximum of that function. Describe the graph of the linear approximation.
-
A handrail, which weighs 120 N and is 1.8 m long. was mounted to a wall adjacent to a small set of steps (Figure P4.26). The support at A has broken, and the rail has fallen about the loose bolt at 8...
-
The wavenumber of the incident radiation in a Raman spectrometer is 20 623 cm 1 . What is the wavenumber of the scattered Stokes radiation for the J=42 transition of 16 O 2 ?
-
Use appropriate electronic structure software to perform calculations on H 2 O and CO 2 with basis sets of your or your instructors choosing. (a) Compute ground-state energies, equilibrium geometries...
-
A 5.00mm cell was filled with a solution of a dye. The concentration of the dye was 18.5mmol dm 3 . Calculate the molar absorption coefficient of the dye at this wavelength given that the...
-
question 6 Timely Inc. produces luxury bags. The budgeted sales and production for the next three months are as follows july. august september Sales, in units 1,115. 1229. 1302 Production. in units...
-
On May 12 Zimmer Corporation placed in service equipment (seven-year property) with a basis of $220,000. This was Zimmer's only asset acquired during the year. Calculate the maximum depreciation...
-
Power Manufacturing has equipment that it purchased 7 years ago for $2,550,000. The equipment was used for a project that was intended to last for 9 years and was being depreciated over the life of...

Study smarter with the SolutionInn App


