The concentration dependence of the osmotic pressure of solutions of a macromolecule at 20C was found to
Question:
The concentration dependence of the osmotic pressure of solutions of a macromolecule at 20°C was found to be as follows:
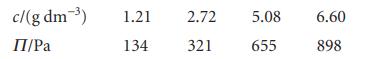
Determine the molar mass of the macromolecule and the osmotic virial coefficient.
Transcribed Image Text:
c/(g dm-3) П/Pa 1.21 134 2.72 321 5.08 655 6.60 898
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
Solution P RV NB where N is the number of particles in the solution B is a consta...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A major airline manufacturer was found to be in violation of FAA safety rules and was forced to install additional safety devices in each of its planes within the next six months. The airline company...
-
A major airline manufacturer was found to be in violation of FAA safety rules and was forced to install additional safety devices in each of its planes within the next six months. The airline company...
-
A major airline manufacturer was found to be in violation of FAA safety rules and was forced to install additional safety devices in each of its planes within six months. The airline company projects...
-
Which statement does NOT reflect a way that journals require authors to disclose potential or actual conflicts of interest? Group of answer choices Require researcher's Federal tax statements Require...
-
The following hypotheses are to be tested: H0: p 0.65 HA: p > 0.65 A random sample of 500 is taken. Using each set of information following, compute the power of the test. a. = 0.01, true p = 0.68...
-
Identify each equation. If it is a parabola, give its vertex, focus, and directrix; if an ellipse, give its center, vertices, and foci; if a hyperbola, give its center, vertices, foci, and...
-
Suppose two assets satisfy a statistical factor model with a single factor: R 1 = E[R 1] + f + 1 , R 2 = E[R 2] f + 2, where E[f] = E[ 1] = E[2] = 0, var(f) = 1, cov(f ,1) = cov(f ,2) = 0, and...
-
On March 31, 2016, the following data were accumulated to assist the accountant in preparing the adjusting entries for Potomac Realty: a. The supplies account balance on March 31 is $5,620. The...
-
[Mortgage] What is the monthly payment on a 30-year mortgage of $100,000 at 8% interest per year, compounded monthly? What is the total amount paid on this loan over the 30-year period?
-
Your tax client, Steve Michaels, told you that his former accountant who prepared his annual tax returns made errors that resulted in him suffering more than $100,000 in losses. Apparently, the...
-
It is observed that the critical micelle concentration of sodium dodecyl sulfate in aqueous solution decreases as the concentration of added sodium chloride increases. Explain this effect.
-
What is the relative rate of sedimentation for two spherical particles of the same density, but which differ in radius by a factor of 10?
-
Two beakers, one containing 0.010 m NaCl(aq) and the other containing pure water, are placed inside a bell jar and sealed. The beakers are left until the water vapor has come to equilibrium with any...
-
"The initial speed with which a ball is thrown is doubled, with the angle of projection fixed. Is the maximum height to which the ball rises doubled?" Now, let's say you are also allowed to change...
-
Wally Working Co. emiti bonos con una tasa de inters nominal (contratada) de 15%, por un valor ominal de $80,000, con un vencimiento de 5 anios. Cuando emiti los bonos, la tasa de inters del mercado...
-
Using the Central Limit Theorem. In Exercises 5-8, assume that the amounts of weight that male college students gain during their freshman year are normally distributed with a mean of 1.2 kg and a...
-
Swain Athletic Gear (SAG) operates six retail outlets in a large Midwest city. One is in the center of the city on Cornwall Street and the others are scattered around the perimeter of the city....
-
Please help Calculating NPV and IRR Businesses use NPV and IRR to determine whether a project will add - value for shareholders. After watching the CFA Level I Corporate Finance video, answer the...
-
Evaluate the integral, if it exists. 10 dx x2 4
-
The Cholesterol Level data sets give cholesterol levels of heart attack patients. Cholesterol measures are taken 2, 4, and 14 days aft er a patient has suffered a heart attack. Is there a significant...
-
A molecule in a gas undergoes about 1.0 X 109 collisions in each second. Suppose that (a) Every collision is effective in deactivating the molecule rotationally and (b) That one collision in 10 is...
-
Calculate the frequency of the J = 3 f-- 2 transition in the pure rotational spectrum of 12CI60. The equilibrium bond length is 112.81 pm.
-
If the wave number of the J = 1 f-- 0 rotational transition of IH81Br considered as a rigid rotator is 16.93 cm-1, what is (a) The moment of inertia of the molecule, (b) The bond length?
-
Machinery is purchased on May 15, 2015 for $120,000 with a $10,000 salvage value and a five year life. The half year convention is followed. What method of depreciation will give the highest amount...
-
Flint Corporation was organized on January 1, 2020. It is authorized to issue 14,000 shares of 8%, $100 par value preferred stock, and 514,000 shares of no-par common stock with a stated value of $2...
-
Question 24 Not yet answered Marked out of 1.00 P Flag question Muscat LLC's current assets and current liabilities are OMR 258,000 and OMR 192,000, respectively. In the year 2020, the company earned...

Study smarter with the SolutionInn App


