The following data have been obtained for the adsorption of H2 on the surface of 1.00 g
Question:
The following data have been obtained for the adsorption of H2 on the surface of 1.00 g of copper at 0°C. The volume of H2 below is the volume that the gas would occupy at STP (0°C and 1 atm).
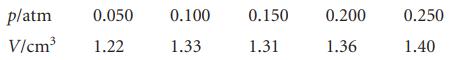
Determine the volume of H2 necessary to form a monolayer and estimate the surface area of the copper sample. The density of liquid hydrogen is 0.708 g cm−3.
Transcribed Image Text:
p/atm 0.050 V/cm³ 1.22 0.100 1.33 0.150 1.31 0.200 1.36 0.250 1.40
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (10 reviews)
Solution The gas adsorption isotherm is given by In the above equ...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following data have been obtained for the adsorption of H 2 on the surface of 1.00 g of copper at 0 C. The volume of H2 below is the volume that the gas would occupy at STP (0 C and 1 atm)....
-
The following data have been obtained for the liquid-vapour equilibrium compositions of mixtures of nitrogen and oxygen at 100 kPa. T/K 77.3 78 80 82 84 86 88 90.2 X (O2) 0 10 34 54 70 82 92 100...
-
Following data have been obtained for machining AA390 Aluminum, a Si-Al alloy. Compute the K and n values for the Taylor tool life equation. How do these n values compare to the typical values?...
-
Please help me calculate the current assets and current liabilities. Cash and cash equivalents Deposits Marketable securities Inventory Property & equipment, net Loan to shareholders Notes receivable...
-
The manager of the Cottonwood Grille recently selected a random sample of 18 customers and kept track of how long the customers were required to wait from the time they arrived at the restaurant...
-
Find the partial fraction decomposition for the rational expression. x(x + 3)
-
What are the roles of comparative and competitive advantages in Unilevers success? Provide specific examples of natural and acquired advantages that Unilever uses to succeed in the global FMCG...
-
The adjusted trial balance of Hodges Company shows these data pertaining to sales at the end of its fiscal year, October 31, 2014: Sales Revenue $900,000; Freight-Out $14,000; Sales Returns and...
-
Equipment (3) 29,700 Dividends (9) 4,100 Cash (1) 41,300 (2) 2,900 (7) 16,500(3) 5,350 (4) 4,550 (6) 12,400 (9) 4,100 Accounts Receivable (5) 22,300 (7) 16,500 Accounts Payable (6) 12,400 (3) 24,350...
-
Scorchys Tacos restaurant is considering expanding into suburban markets in Houston, Texas, with three new locations. Because of the floods in 2017, many restaurants never reopened, creating a...
-
The enthalpy of adsorption of CO on a surface is found to be 120 kJ mol 1 . Estimate the mean lifetime of a CO molecule on the surface at 400 K.
-
The volume of oxygen gas at 0C and 101 kPa adsorbed on the surface of 1.00 g of a sample of silica at 0C was 0.284 cm 3 at 142.4 Torr and 1.430 cm 3 at 760 Torr. What is the value of V mon ?
-
Part 1: On July 1, 2022, Wallace Company, a calendar-year company, sold specialorder merchandise on credit and received in return an interest-bearing note receivable from the customer. Wallace...
-
The answer above is NOT correct. The value of (2x + 1)(x + x)dx is
-
Review the resource on organizational theory. Explore the various theories and select one to use for this Discussion. Consider the strengths and limitations of the chosen theory. Compose an analysis...
-
How do the locations of Australian department store Myer affect the ability of the other factors of the operating model canvas (suppliers, organization, processes, and information/management systems)...
-
Critical Reading Review: The Exclusion of Latinos from American Media and History Books Read the article. After reading the article, answer the following questions: 1. What purpose do you think the...
-
1. How does the proposed market segment of residential contracts differ from Smith Electric's current market segment? 2.What does a SWOT analysis tell us about Smith Electric's ability to enter a...
-
A particle moves along a straight line with equation of motion s = f (t), where s is measured in meters and t in seconds. Find the velocity and the speed when t = 4. f (t) = 80t - 6t 2
-
TRUE OR FALSE: 1. Banks with a significantly large share of fixed-interest rate home loans are less exposed to interest rate risks. 2. Although Australian banks are pretty big, they are not...
-
Which of the following systems are isolated? a) A bottle of wine b) A tightly sealed, perfectly insulated thermos bottle c) A tube of toothpaste d) our solar system. Explain your answers.
-
Why do the z and y components of the velocity not change in the collision depicted in Figure 1.2? Figure 1.2 mvx mvx
-
A mixture of 2.10 10 3 g of O 2 , 3.88 10 -3 mol of N 2 , and 5.25 10 20 molecules of CO are placed into a vessel of volume 5.25 L at 12.5C. a. Calculate the total pressure in the vessel. b....
-
On February 1, 2021, Arrow Construction Company entered into a three-year construction contract to build a bridge for a price of $8,600,000. During 2021, costs of $2,200,000 were incurred with...
-
Salespersons' Report and Analysis Walthman Industries Inc. employs seven salespersons to sell and distribute its product throughout the state. Data taken from reports received from the salespersons...
-
Stockholders do not have the power to bind the corporation to contracts. This is referred to as lack of mutual agency. True false question. True False

Study smarter with the SolutionInn App


