The half-life (t 1/2 ) of a radioactive species is the time it takes for half of
Question:
The half-life (t1/2) of a radioactive species is the time it takes for half of the species to emit radiation and decay (turn into a different species). If a quantity N0 of the species is present at time t = 0, the amount present at a later time t is given by the expression
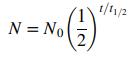
Each decay event involves the emission of radiation. A unit of the intensity of radioactivity is a curie (Ci), defined as 3.7x1010 decay events per second.
A 300,000-gallon tank has been storing aqueous radioactive waste since 1945. The waste contains the radioactive isotope cesium-137 (137Cs), which has a half-life of 30.1 years and a specific radioactivity of 86.58 Ci/g. The isotope undergoes beta decay to radioactive barium-137, which in turn emits gamma rays and decays to stable (nonradioactive) barium with a half-life of 2.5 minutes. The concentration of 137Cs in 2013 was 2.50x10-3 g/L.
(a) What fraction of the 137Cs would have to decay for the level of cesium-related radioactivity of the contents to be 1.00x10-3 Ci/L? What total mass of cesium (kg) would that loss represent? In what year would that level be reached?
(b) What was the concentration of 137Cs in the tank (g/L) when the waste was first stored?
(c) Explain why the radioactive cesium in the tank poses a significant environmental threat while the radioactive barium does not.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





