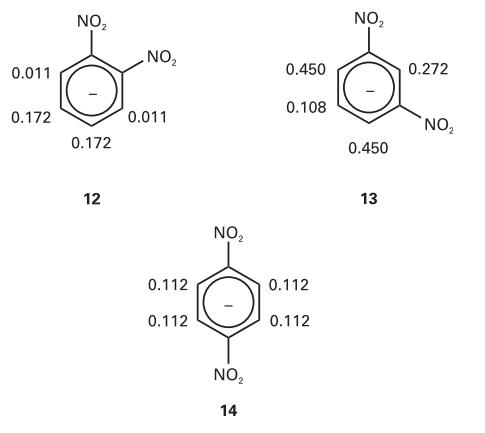
The hyperfine coupling constants observed in the radical anions (12), (13), and (14) are shown (in millitesla,
Question:
The hyperfine coupling constants observed in the radical anions (12), (13), and (14) are shown (in millitesla, mT). Use the value for the benzene radical anion to map the probability of finding the unpaired electron in the π orbital on each C atom.
Transcribed Image Text:
0.011 0.172 NO ₂ 0.172 12 NO₂ 0.011 0.112 0.112 NO ₂ NO ₂ 14 0.450 0.108 0.112 0.112 NO₂ 0.450 13 0.272 NO₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
Solution For radical anions hyperfine coupling constants are give...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The hyperfine coupling constant in CH3 is 2.3 mT. Use the information in Table 15.3 to predict the splitting between the hyperfine lines of the spectrum of CD what are the overall widths of the...
-
The hyperfine interaction in a hydrogen atom between the magnetic dipole moment of the proton and the spin magnetic dipole moment of the electron splits the ground level into two levels separated by...
-
The relative orbital levels for the hydrogen atom can be represented as Draw the relative orbital energy levels for atoms with more than one electron, and explain your answer. Also explain how the...
-
Prove that, the area of the traverse is equal to the algebraic sum of the products of the total latitude of each point and algebraic sum of the departures of the lines meeting at that point.
-
Discussion Questions: 1. After returning from the training session at Hamburger University, a McDonalds store owner selected a random sample of 362 drive-thru customers and carefully measured the...
-
Use the properties of logarithms to rewrite each expression. Simplify the result if possible. Assume all variables represent positive real numbers. 6 log, 62
-
In what way does your program of studies contribute to building your self-confidence and self-esteem?
-
1. Does Cold Stone Creamery represent a high performance approach to planning? Why or why not? 2. Cold Stones Pyramid of Success 2010 represents an example of a single use plan. What might be a...
-
E 1 1 . 4 ( L 0 1 , 2 , 4 ) Company. The following is selected information for Alatorre Alatorre purchased a patent from Vania Co . for $ 1 , 0 0 0 , 0 0 0 on January 1 , 2 0 2 3 . The patent is...
-
Consider a closed vessel initially containing 1 mol of pure tetrahydrofuran at 74C and 120 kPa. Imagine that pure chloroform is slowly added at constant temperature and pressure until the vessel...
-
Sketch the appearance of the 1 H-NMR spectrum of acetaldehyde (ethanal) using J = 2.90 Hz and the data in Exercise 15.9a in a spectrometer operating at (a) 250 MHz, (b) 500 MHz. Data in Exercise...
-
The chemical shift of the CH 3 protons in acetaldehyde (ethanal) is = 2.20 and that of the CHO proton is 9.80. What is the difference in local magnetic field between the two regions of the molecule...
-
Discover the referent people or groups and perceived outcome/input ratios for employees equity computation; compare your assessments of likely equity with theirs. lop5
-
MTB Surfboards has a P / E of 2 0 . The discount rate for this firm is 3 0 percent. They had earnings of $ 2 , 0 0 0 , 0 0 0 and 1 0 0 , 0 0 0 shares of common stock outstanding. What should be the...
-
Question 4 (20 marks) Laboratory 4: Superposition Theorem Objectives: 1. Understand the principles of a Superposition Theorem 2. Determine the characteristics of a Superposition Theorem...
-
2 Ursala, Inc., has a target debt-equity ratio of .65. Its WACC is 10.4 percent, and the tax rate is 23 percent. a. If the company's cost of equity is 14 percent, what is its pretax cost of debt? b....
-
Thinking about Nike's corporate practices, discuss your approach to starting a company that outsourced labor in order to reduce manufacturing costs. What decisions would you make to combine...
-
Owen Properties recently purchased a building in a community that is eligible for participation in the National Flood Insurance Program (NFIP). Under the regular program of the NFIP, the maximum...
-
Nancy has active modified adjusted gross income before passive losses of $75,000. She has a loss of $5,000 on a rental property she actively manages. How much of the loss is she allowed to take...
-
Write a program that initializes an array. It inputs a value from the user and searches the number in the array.
-
The dissociation vapour pressure ofNH4Cl at 427C is 608 kPa but at 459C it has risen to 1115 kPa. Calculate (a) The equilibrium constant, (b) The standard reaction Gibbs energy, (c) The standard...
-
Estimate the temperature at which CuS045H,O undergoes dehydration.
-
For PbI2(s) = 0Pb+(aq) + 2 r(aq), K = 1.4 X 10-8 at 25C and the standard Gibbs energy of formation ofPbI2(s) is -173.64 k] mol ". Calculate the standard Gibbs energy of formation of PbI2 (aq).
-
How does budgeting household expenses differ from budgeting business expenses? What are the similarities?
-
This is a partial adjusted trial batance of Cullumber Compary manualys
-
Which of the following journal entries will record the payment of a $1,500 salaries payable originally incurred for Salaries Expense? Select one: A. Debit Salaries Expense; credit Salaries Payable B....

Study smarter with the SolutionInn App


