The observed lines in the emission spectrum of atomic hydrogen are given by In the notation favored
Question:
The observed lines in the emission spectrum of atomic hydrogen are given by
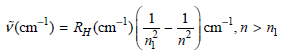
In the notation favored by spectroscopists, ν̅ = 1/λ = E/hc and RH =109,677 cm−1. The Lyman, Balmer, and Paschen series refers to n1 = 1, 2, and 3, respectively, for emission from atomic hydrogen. What is the highest value of ν???? and E in each of these series?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





