Two empirical equations of state of a real gas are as follows: Evaluate (S/V) T for each
Question:
Two empirical equations of state of a real gas are as follows:
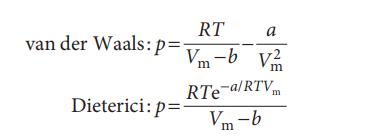
Evaluate (∂S/∂V)T for each gas. For an isothermal expansion, for which kind of gas (also consider a perfect gas) will ΔS be greatest? Explain your conclusion.
Transcribed Image Text:
RT a van der Waals: p=V-b V m m RTe-a/RTVm Dieterici: p= V-b
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
For the van der Waals gas we have SV pVT pTV SV RTVmbVm2bVm aRTVm2bVm SV ...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula
Question Posted:
Students also viewed these Sciences questions
-
Consider the isothermal expansion of 1.00 mole of ideal gas at 27oC. The volume increases from 30.0 L to 40.0 L. Calculate q, w, E, H, S, and G for two situations: a. a free expansion b. a reversible...
-
A quantity of an ideal gas undergoes an isothermal expansion at 20 oC and does 3.0 x 103 J of work on its surroundings in the process. (a) Will the entropy of the gas (1) increase, (2) remain the...
-
Explain how the perfect gas equation of state arises by combination of Boyle's law, Charles's law, and Avogadro's principle.
-
What do you think people would say about Corrie from the few quotes we have from her book? What was her personality like? Do you think she handled her incarceration differently than Elie Wiesel?...
-
Summa Manufacturing Company issued $ 900,000 par value, 5%, five- year bonds dated January 1, 2016. The bonds pay interest semiannually each June 30 and December 31. Summa issued the bonds on April...
-
Sketch the n = 8 wave function for the potential energy shown in Figure EX 40.12. U(x) Es
-
Ranging behavior of Spanish cattle. The cattle inhabiting the Biological Reserve of Doana (Spain), live under free-range conditions, with virtually no human interference. The cattle population is...
-
Find the amount to which $500 will grow under each of these conditions: a. 12% compounded annually for 5 years b. 12% compounded semiannually for 5 years c. 12% compounded quarterly for 5 years d....
-
The following information comes from the 2 0 2 4 Annual Report to stockholders of Composition Incorporated ( in thousands ) : From the Statement of Changes in Shareholders' Equity: From the Statement...
-
Colton Enterprises experienced the following events for Year 1, the first year of operation: 1. Acquired $35,000 cash from the issue of common stock. 2. Paid $12,000 cash in advance for rent. The...
-
Which of F 2 (g) and I 2 (g) is likely to have the higher standard molar entropy at 298K?
-
Discuss the relationships between the various formulations of the Second Law of thermodynamics.
-
Data processing activities may be classified in terms of three stages or processes: input, processing, and output. An activity that is not normally associated with the input stage is a. batching. b....
-
For the data in Problem 42, how would you predict demand for medical kits using (a) moving averages and (b) exponential smoothing (with alpha values equal to 0.5 and greater) for the 21st week? Data...
-
For a light ray that crosses the interface between medium 1 having index of refraction \(n_{1}\) and medium 2 having index of refraction \(n_{2}\), what relationship between \(\theta_{1}\) and...
-
The atmosphere of the planet Venus is almost entirely composed of carbon dioxide (about 96.5 % carbon dioxide). The carbon dioxide on Venus might be in equilibrium with carbonate ions in minerals on...
-
Seniority quantum numbers typically measure how many fermions are in some sense "not paired" with another fermion. For the quasispin model of Problem 31.3 , define the Racah seniority $v$ through...
-
(a) Place a perfectly conducting sphere with radius a in a uniform electric field E 0 and let an origin centered electric dipole field represent the field produced by the sphere. Use this information...
-
Does the production of a hologram involve the interference of light waves? Explain.
-
A copper sphere of 10-mm diameter, initially at a prescribed elevated temperature T;, is quenched in a saturated (1 atm) water bath. Using the lumped capacitance method, estimate the time for the...
-
Discuss the steps involved in the construction of sp 3 , sp 2 , and sp hybrid orbitals.
-
Give the ground-state electron configurations and bond orders of (a) Li 2 , (b) Be 2 , and (c) C 2 .
-
The overlap integral between two H1 s orbitals on nuclei separated by a distance R is S = {1 + (R/a 0 ) + 1/3 (R/a 0 ) 2 }e R/a0 . Plot this function for 0 R < .
-
On April 1, year 1, Mary borrowed $200,000 to refinance the original mortgage on her principal residence. Mary paid 3 points to reduce her interest rate from 6 percent to 5 percent. The loan is for a...
-
Give a numerical example of: A) Current liabilities. B) Long-term liabilities?
-
Question Wonder Works Pte Ltd ( ' WW ' ) produces ceramic hair curlers to sell to department stores. The production equipment costs WW $ 7 0 , 0 0 0 four years ago. Currently, the net book value...

Study smarter with the SolutionInn App


