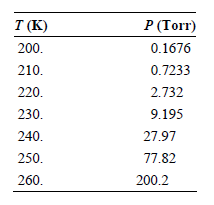
Use the vapor pressures of ice given here to calculate the enthalpy of sublimation using a graphical
Question:
Transcribed Image Text:
T (K) P (Torr) 200. 0.1676 210. 0.7233 2.732 220. 230. 9.195 240. 27.97 250. 77.82 200.2 260.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
A least squares fit of ...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the vapor pressures of SO 2 (l) given in the following table to calculate the enthalpy of vaporization using a graphical method or a least squares fitting routine. T (K) 190. P (Pa) T (K) 230. P...
-
Use the vapor pressures of n-butane given in the following table to calculate the enthalpy of vaporization using a graphical method or a least squares fitting routine. P (Pa) 1000 x 104 1000 x 105 T...
-
Use the vapor pressures of tetrachloromethane given in the following table to calculate the enthalpy of vaporization using a graphical method or a least squares fitting routine. P/Pa T (K) 320. 330....
-
A Norman window has the outline of a semicircle on top of a rectangle. Suppose there are 8 + feet of wood trim available. Discuss why a window designer might want to maximize the area of the window....
-
Select 4 similar size public companies in the same industry. The company's size can be measured by total assets or total sales. Obtain the most recent financial statements of these companies, apply...
-
Derive a conversion factor relating pressure in pascals (N/m 2 ) and in psi (lbf/in 2 ). Compare your result with the conversion factor at the front of this book.
-
Aedion Company owns control over Breedlove, Inc. Aedion reports sales of $300,000 during 2009 and Breedlove reports $200,000. Inventory costing $20,000 was transferred from Breedlove to Aedion...
-
Discuss how technology and the emerging role of digital and social media are impacting the role of brand managers. What types of skills are needed to be a successful brand manager today? What can...
-
Selling price of home Down payment Principal (loan) Rate of interest Years Payment per $1,000 Monthly mortgage payment $96,000 $5,000 5.5 % 30
-
Project Alpha has two phases. You may invest in the first, in both, or in neither. The first phase requires an investment of $100 today. One year later, Alpha will deliver either $120 or $80, with...
-
The phase diagram of NH 3 can be characterized by the following information. The normal melting and boiling temperatures are 195.2 and 239.82 K, respectively; the triple point pressure and...
-
Calculate the vapor pressure for a mist of spherical water droplets of radius a. 1.95 10 8 m b. 2.25 10 6 m at 298 K. The vapor pressure of water at this temperature is 25.2 Torr.
-
Calculate the magnitude and location of the vertical and horizontal components of the hydrostatic force on the surface shown in Figure P2.6.9 (quadrant on top of the triangle, both with a unit...
-
3. Different positions of Vermiform appendix.
-
Define Image?
-
What is Dynamic Range?
-
Define Brightness?
-
What is Chromatic Adoption?
-
The cooling system of a certain foreign-made car has a capacity of 15 liters. If the system is filled with a mixture that is 40% antifreeze, how much of this mixture should be drained and replaced by...
-
Consider the reaction of acetic acid in water CH 3 CO 2 H(aq) + H 2 O(l) CH3CO 22 (aq) + H 3 O + (aq) where Ka 5 1.8 3 1025. a. Which two bases are competing for the proton? b. Which is the stronger...
-
Find the gradient of the function g(x,y,z) = ax 3 + ye bz , where a and b are constants.
-
Find r where r = ix + jy + kz.
-
Find the Laplacian of the function f = exp (x 2 + y 2 + z 2 ) = e x2 e y2 e z2 .
-
Your company produces a health magazine. Its sales data for 1 - year subscriptions are as follows: Year of Operation Subscriptions Sold % Expired at Year End 2 0 2 0 $ 3 0 0 , 0 0 0 5 2 0 2 1 $ 6 4 7...
-
Problem 3 - 2 0 ( Static ) Calculate profitability and liquidity measures LO 3 - 3 , 3 - 4 , 3 - 6 Presented here are the comparative balance sheets of Hames Incorporated at December 3 1 , 2 0 2 3...
-
3 Required information [The following information applies to the questions displayed below) John and Sandy Ferguson got married eight years ago and have a seven-year-old daughter. Samantha. In 2020,...

Study smarter with the SolutionInn App


